Consider CGRP Inhibitors as a First-Line Option for Preventive Treatment of Migraine: New American Headache Society Consensus Statement
The AHS recommends the migraine-specific preventive medications be prescribed first, with no requirements to "fail" other treatments for eligibility.
The CGRP-targeting migraine therapies are a first-line option for migraine prevention. Initiation of these therapies should not require trial and failure of non-specific migraine preventive medication approaches.
This position statement from the American Headache Society (AHS) is the first of its kind from the organization calling for the calcitonin gene-related peptide (CGRP)-targeting therapies to be selected over traditional nonspecific agents used as first line preventive migraine treatment without requiring the time and frustration of the “trial and failure” protocol currently considered standard of care.
Preventive therapy is indicated in approximately 40% of individuals with migraine, according to the AHS, but only about 10% of those use the treatments. The reasons include longstanding limitations with both efficacy and tolerability of the more established therapies, according to Andrew Charles, MD, statement co-author and president of the American Headache Society, and colleagues .
The new consensus statement, published in March in the journal Headache, is based on a comprehensive review by the AHS of clinical trial and real-world experience supporting the efficacy, tolerability, and safety of the CGRP inhibitors for migraine prevention, a body of evidence that supports the AHS consensus authors’ statement that CGRP-targeting therapies have “rapidly become an indispensable option for the prevention of migraine.” Following is a summary of the key evidence the AHS points to as support for the updated statement.
Brief background
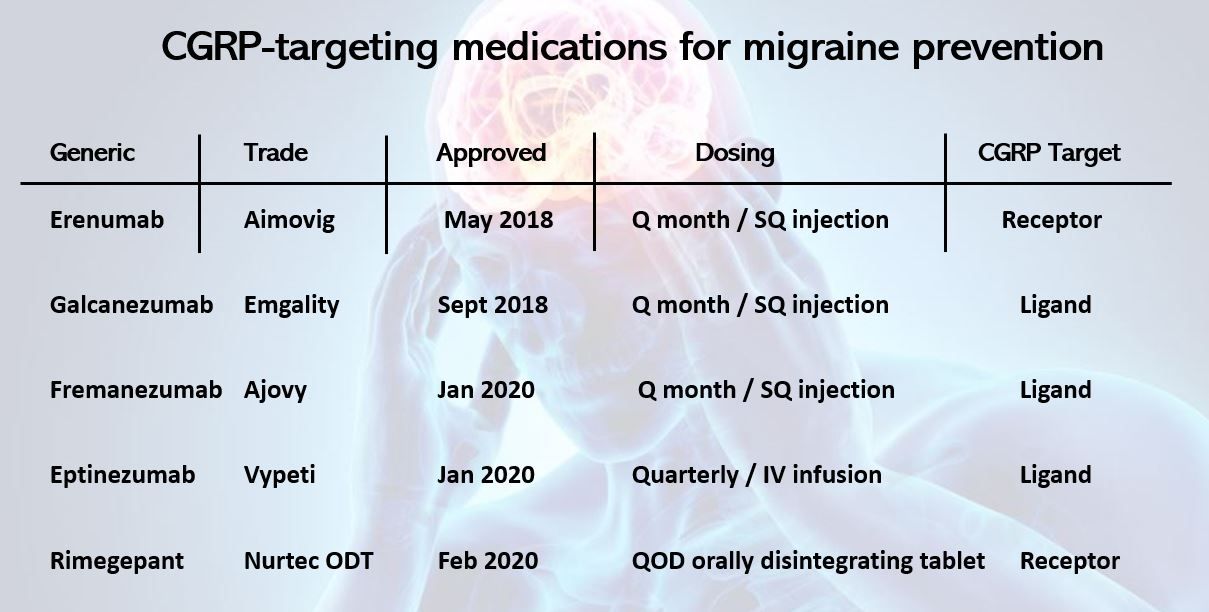
The first CGRP inhibitor for migraine prevention, erenumab, was approved by the US Food and Drug Administration (FDA) in May 2018 and was followed in rapid succession by approvals for galcanezumab (September 2018), and fremanezumab and eptinezumab, both in January 2020. In addition, the FDA has approved 2 oral small molecule CGRP receptor antagonists (gepants) rimegepant (February 2020) and atogepant (September 2021).
©yodiyim/stock.adobe.com
The previous AHS statement on migraine treatment recommended that an individual try at least 2 classes of the medications considered first-line therapy at the time for 8 weeks or more before they could be considered for CGRP-targeting therapy. A trial of onabotolinumtoxin A could stand for an alternative to a trial of 2 classes of medications. The requirement for sequential trial and error, or step therapy, also has been incorporated into the approval of the CGPR-targeting drugs for insurance coverage. AHS consensus statements have committed to provide “iterative updates as evidence and experience accumulated,” the authors wrote, and the evolution in the approach to migraine-specific preventive therapy provides the basis for the current update.
"Moving CGRP-targeting therapies to the first line of treatment could have a transformational impact on the prevention of migraine attacks and their associated burdens," Charles said in a statement from AHS.2 "Elevating CGRP-targeting therapies to the first line should reduce barriers for patients to receive these effective treatments and bring hope to countless people who experience this invisible, yet debilitating disease."
Migraine-specific, broad evidence base
A foundation of significant and expanding evidence has established the neuropeptide CGRP as playing a fundamental role in the etiology of migraine, paving the way for research and discovery of targeted therapies that are “migraine specific.” The traditional first line migraine-preventive options came from a range of classes, including antihypertensives, antiseizure medications, and antidepressants, the AHS wrote. Consequently, the choice of which to try first has often been driven by the presence of comorbidities that could potentially benefit from the treatment or that could contraindicate the drug. Nor could a clinician draw on any clear, established predictors of treatment response to guide a decision.
Adding to the rationale for the updated consensus is evidence for the CGRP inhibitors in a variety of categories beyond the standard endpoint of impact on number of migraine days, Charles and coauthors wrote. Those categories include responder rates, use of acute rescue medication, efficacy in patients with multiple prior treatment failures, efficacy in those with and without migraine aura, and impact on migraine-related disability. None of these categories have been evaluated for the nonspecific traditional preventive therapies.
The fact that all the current CGRP-targeting agents (save 1) are approved by the US FDA for prevention of both chronic and episodic migraine sets them apart, again, from current treatment options. This feature, unique to the class, can enhance flexibility for health care professionals and for patients whose headaches may shift spontaneously along the continuum of episodic and chronic migraine, AHS said.
"Real-world" experience, cost
“Real-world” studies of the experience with CGRP inhibitors have been conducted worldwide, according to AHS, and while these analyses generate a different type of evidence, their findings have generally corroborated those from randomized controlled clinical trials in terms of safety, efficacy, and tolerability. Safety concerns reported from such studies include constipation and hypertension (reported mainly with erenumab), and Raynaud phenomenon, said AHS, but to date they appear to be uncommon, and it is rare for them to lead to discontinuation of therapy. Regarding the long-term safety of CGRP-targeting therapies, continued expansion of use and ongoing post-marketing surveillance “increases the confidence that any adverse effects…will be identified rapidly…and addressed appropriately,” AHS wrote.
In recommending that CGRP-targeting therapies be considered a first-line option for migraine prevention, AHS acknowledges their annual cost as significantly greater than the majority of nonspecific preventives (most are available as generic formulations) and that the “cost-benefit” relationship of a new treatment compared with an existing one poses a number of challenges. While the organization said it cannot justify manufacturer’s pricing, the statement enumerates the costs to the individual, the health care system and to society of delayed and inadequate treatment for migraine and outlines benefits of treatment with CGRP inhibitors that have no corresponding examples among the previous first-line treatments.
"It is clear that if cost were not a primary consideration, there would be no controversy regarding the legitimate place for CGRP-targeting therapies as a first-line option for migraine prevention given their established safety, efficacy, and years of integration into practice," Charles et al wrote. "While some head-to-head evidence and substantial ‘real-world’ experience indicates that CGRP-targeting therapies may be a superior option for a significant number of patients, further evidence and experience are needed to conclude that CGRP-targeting therapies are the first-line therapy, as opposed to a first-line therapy option, as is the position of this updated consensus statement from the AHS," they concluded.
References
1. Charles AC, Digre KB, Goadsby PJ, Robbins MS, Hershey A. Calcitonin gene-related peptide-targeting therapies are a first-line option for the prevention of migraine: an American Headache Society position statement update. Headache. Published March 11, 2024. doi:10.1111/head.14692
2. American Headache Society publishes updated guidance on migraine preventive therapy. News release. American Headache Society. March 11, 2024. Accessed April 11, 2024. https://www.prnewswire.com/news-releases/american-headache-society-publishes-updated-guidance-on-migraine-preventive-therapy-302085761.html
Newsletter
Enhance your clinical practice with the Patient Care newsletter, offering the latest evidence-based guidelines, diagnostic insights, and treatment strategies for primary care physicians.