
Pediatrics
Latest News
Latest Videos
CME Content
More News

The VITESSE trial met its primary endpoint, with the VIASKIN Peanut patch improving desensitization versus placebo in peanut-allergic children aged 4 to 7 years.

CDC officials say the shift reflects evidence review and informed consent, as public health groups warn of potential downstream effects.
The inquiry follows internal requests for closer review of trial findings and real-world evidence; regulators have not yet indicated any planned actions.

Chari A. Cohen, DrPH, MPH, underscored the importance of trust, open dialogue, and evidence-based information.
Amid controversy, ACIP voted today to end the universal hepatitis B birth dose for infants of HBsAg-negative mothers, shifting to clinician-guided individual decision-making.

Adolescents acquiring smartphones at 12 showed increased risk for depression, obesity and sleep disturbances, according to a study of more than 10,000 youths.

Emotional red flags in AD, eg, sleep loss, isolation, hopelessness, often go unseen. Quick screening questions can surface the real burden individuals carry.

A 2-fold rise in pediatric hypertension over 2 decades and new long-term imaging data signal a need to rethink how early clinicians act on elevated BP.

FDA reviews roflumilast cream for young children with plaque psoriasis, potentially offering a first-of-its-kind topical treatment option.
ACAAI 2025. Phase 3 data show tapinarof cream provides early, sustained improvement and good tolerability in children aged ≥2 years with atopic dermatitis.

Sublingual epinephrine film matches adult PK/PD in kids aged 7-17 with a history of allergic reactions and high risk for serious allergic reactions, incluing anaphylaxis.

Your daily dose of the clinical news you may have missed.

Your daily dose of the clinical news you may have missed.

Nonstimulant medications play a crucial role in ADHD treatment, offering effective alternatives for patients intolerant to stimulants or at risk of misuse.

The sBLA includes data from the phase 3 INHALE-1 study, which showed Afrezza noninferior to MDI insulin. A PDUFA date is set for May 29, 2026.

The approval of roflumilast 0.05% provides a steroid-free, once-daily treatment option for safe and effective long-term management of pediatric eczema.
Measles vaccine coverage must reach 93% to maintain herd immunity but in 1 postelimination outbreak, the level reached only 80% in school-aged children.

The FDA has approved guselkumab for children aged 6 years and older with moderate to severe plaque psoriasis or active psoriatic arthritis, making it the first IL-23 inhibitor authorized for pediatric use.
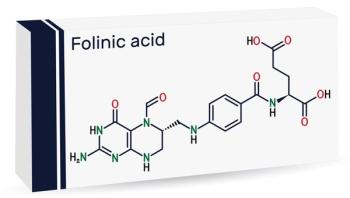
The FDA has initiated approval of leucovorin calcium tablets for children with cerebral folate deficiency, which can manifest with autistic features. What is it?
Corticosteroids following resolution of anaphylaxis should be chosen with clear, clinical rationale and reserved for select children, study authors concluded.

Incyte announced the agency's decision, which was based on findings of significant reduction in atopic dermatitis signs and symptoms during the TRuE-AD3 clinical trial.

EADV 2025: New phase 3 data reveal delgocitinib cream's efficacy for chronic hand eczema in adolescents, confirming its safety profile in adults.

In children aged 10 to <18 with T2D inadequately controlled on standard of care treatment, tirzepatide reduced HbA1c by an average of 2.2% and reduced BMI as well.
Most parents support childhood vaccines such as MMR and polio, but skepticism about seasonal and newer shots persists, along with distrust of federal agencies.

Merck's CAPVAXIVE could cover approximately 78% of invasive pneumococcal disease cases in youth with chronic health conditions, vs other approved pneumococcal conjugate vaccines.





































































































































