Congestive Heart Failure Therapy:
ABSTRACT: Recent studies, although suggestive, do not yet support the routine use of angiotensin II receptor blockers in combination with angiotensin-converting enzyme (ACE) inhibitors in patients with congestive heart failure (CHF). For CHF patients in normal sinus rhythm, consider digoxin when a regimen of diuretics, ACE inhibitors, and β-blockers at optimal dosages does not relieve symptoms completely. Anticoagulation may be warranted in CHF patients with atrial fibrillation, previous embolic events, severely reduced systolic performance, or potential chamber clots. β-Blockers are indicated for patients with mild to severe CHF, unless there is a specific contraindication, and therapy should be initiated once euvolemia has been achieved. Avoid NSAIDs and cyclooxygenase-2 inhibitors in patients with CHF because the prostaglandin-blocking properties of these agents may promote fluid retention.
Five million Americans have congestive heart failure (CHF); each year, there are at least 2 million hospitalizations for this disease. These numbers reflect a growing epidemic, principally among the elderly.
Many CHF patients are alive because of excellent reperfusion treatments for myocardial infarction, antiarrhythmic therapies, surgery for heart valve disease, and advances in the pharmacologic treatment of hypertension. Many of these "survivors" rely on primary care physicians for ongoing care.
In this article, I address some of the newer issues in the management of patients with CHF.
Is it appropriate to use angiotensin II receptor blockers (ARBs) in combination with angiotensin-converting enzyme (ACE) inhibitors in patients with CHF?
The results of recent studies, al- though suggestive, do not yet sup- port the routine use of this thera- peutic combination.
ACE inhibitors have assumed a key role in CHF therapy based on a large body of evidence from controlled trials that shows improvement in morbidity and mortality (Table). These compounds work by blocking ACE, which changes inactive angiotensin I into the active form, angiotensin II. Angiotensin II then attaches to the angiotensin II receptor, activating vasoconstriction, sympathetic nervous system stimulation, aldosterone release, sodium retention, and vascular as well as cardiac muscular hypertrophy. These unfavorable effects are largely blocked by ACE inhibitors, although not entirely so, because there are non-ACE pathways-for example, those of chymase and other enzymes-by which angiotensin I may be converted to angiotensin II. Thus, direct ARBs may provide fuller inhibition of the effects of angiotensin II.
ACE inhibitors also block the degradation of bradykinin. The resulting accumulation has desirable consequences-such as vasodilatation, which is likely helpful in CHF. It may also have undesirable effects, such as cough, which is intolerable in as many as 20% of patients. ARBs neither augment bradykinin-related vasodilatation nor cause cough and therefore are well tolerated.
Because ARBs have fewer side effects than ACE inhibitors and block the effects of angiotensin II more completely, they have been studied in patients with CHF, either alone or with ACE inhibitors.
In the Evaluation of Losartan in the Elderly (ELITE) study, older patients with CHF were assigned to either the ARB losartan or the ACE inhibitor captopril. There was no significant difference in renal function (the primary end point) between the 2 groups. However, mortality, CHF hospitalization rates, and a composite of both were 32% lower in the ARB group.1 Nevertheless, a follow-up study, ELITE II, failed to show that losartan reduced mortality more than captopril.2 (The reductions were 17.7% and 15.9%, respectively.)
The Valsartan Heart Failure Trial (Val-HeFT) investigated the addition of the ARB valsartan to patients' existing CHF regimen (93% were taking ACE inhibitors and 35% were receiving β-blockers). Most patients had New York Heart Association class II to class IV CHF, and the average left ventricular ejection fraction was 28%.3 Overall mortality was not lowered by the addition of the ARB, but the CHF hospitalization rate and a composite of morbidity and mortality were lower in the valsartan group. Combined morbidity and mortality was 45% lower in ACE inhibitor-intolerant patients who received the ARB, and quality of life, ventricular size, and neurohumoral status (norepinephrine and B-type natriuretic peptide levels) were improved as well.4
These results demonstrated the usefulness of ARBs in CHF patients who could not take an ACE inhibitor. However, patients in the valsartan group who were taking 2 other neurohumoral inhibitors (an ACE inhibitor and a β-blocker) experienced less benefit than those given valsartan with either an ACE inhibitor or a β-blocker alone. This resulted in the recommendation that no more than 2 neurohumoral inhibitors and a diuretic be used in combination.
Is digoxin recommended for patients with CHF who have normal sinus rhythm?
2Digitalis glycosides have been used for CHF for many years. These agents have been viewed as especially beneficial for ventricular rate control in patients whose CHF is complicated by rapid atrial fibrillation. Until intravenous amiodarone became available, digoxin was the only agent available in this setting that was not inotropically negative.
Some authorities have suggested that digoxin is useful in patients with CHF who have normal sinus rhythm because of its positively inotropic properties. Digoxin reduces renal tubular sodium reabsorption, suppresses renin production, and lowers sympathetic outflow from the CNS. Clinical trials show that although the drug improves clinical status and reduces the risk of CHF hospitalization, it provides no long-term mortality benefit.5,6 Other positively inotropic compounds, such as milrinone and vesnarinone, increased mortality in randomized trials.
The reason for the lack of survival benefit with digoxin is unclear; one possible factor is the risk of heart block or arrhythmia, particularly in patients who have renal impairment or electrolyte depletion or those who are taking concomitant β-blockers or amiodarone. For CHF patients who have normal sinus rhythm, you may wish to consider digoxin therapy when an optimal regimen of diuretics, ACE inhibitors, and β-blockers has not provided complete symptomatic relief.
Is anticoagulation indicated in patients with CHF?
3This issue remains unresolved. There are insufficient data to support the use of anticoagulation in CHF patients in sinus rhythm. Many clinicians recommend anticoagulation because of the potential thrombotic risks associated with enlarged hypocontractile cardiac chambers and pooling of blood in the systemic veins. The thromboembolic risk in CHF patients is 1% to 3% annually.7 Anticoagulation is indicated in patients with atrial fibrillation and left ventricular dysfunction.
In the Val-HeFT studies, anticoagulation did not reduce thromboembolic events.8 This finding was contradicted by retrospective subgroup analysis of the Studies of Left Ventricular Dysfunction (SOLVD) trials, which revealed that warfarin was associated with reduced morbidity and mortality in patients with CHF, whether or not they were in atrial fibrillation.9 (SOLVD was primarily a trial of the ACE inhibitor enalapril vs placebo, not an evaluation of anticoagulation.) In neither the Val-HeFT nor the SOLVD trial was the intensity of anticoagulation controlled or reported.
Because of these conflicting retrospective analyses, prospective, randomized, controlled studies are needed. Two trials are now enrolling CHF patients with depressed left ventricular function: the Warfarin Versus Aspirin in Reduced Cardiac Ejection Fraction investigation, and the Warfarin and Antiplatelet Therapy in Chronic Heart Failure trial. The latter study will compare warfarin, aspirin, and clopidogrel.
Until the results of these studies are known, there is no specific recommendation, although a strong consensus would favor anticoagulation in CHF patients with atrial fibrillation, previous embolic events, severely reduced systolic performance, or potential chamber clots on imaging studies.
Is there a preferred β-blocker for patients with CHF, and when should therapy be initiated?
4In a significant reversal, β-blockers have now taken a leading role in CHF therapy after earlier being contraindicated because of concern about negative inotropy. Chronic activation of the sympathetic nervous system in CHF is now believed to be responsible for damage to the heart.
Controlled studies of bisoprolol, carvedilol, and metoprolol have demonstrated reduction in symptoms, hospitalizations, and mortality compared with placebo. Improvement in left ventricular ejection fraction has been shown with carvedilol10 and metoprolol.11 Clinical and survival benefits have been demonstrated for both selective and nonselective agents, as well as those that combine β-blocking and α-blocking vasodilator properties.
β-Blockers are now recommended for all patients who have mild to moderate CHF unless a specific contraindication exists, such as cardiogenic shock, bradycardia with a high degree of heart block, severe emphysema, sustained symptomatic hypotension, or active wheezing associated with bronchospasm.
Recent evidence now favors the use of β-blockers in severe CHF as well. The results of the Carvedilol Prospective Randomized Cumulative Survival study showed a 35% survival benefit with this agent.12 The US Carvedilol Heart Failure Study demonstrated a 65% reduction in overall mortality in patients with less severe CHF.13 Trials of bisoprolol and a long- acting metoprolol compound, metoprolol CR/XL, showed a 34% reduction in overall mortality in moderately severe CHF.14-16 In these protocols, the β1-selective agents (bisoprolol and metoprolol) as well as the β1/β2/α-blocker (carvedilol) provided similar symptom and mortality benefits.
In clinical practice, carvedilol may have an advantage. Its α-blocking property may facilitate initiation of therapy because vasodilatation can neutralize any early tendency toward volume retention.17 Alternatively, this effect can cause hypotension in some patients after the first dose. In diabetic patients, carvedilol was associated with greater insulin sensitivity than metoprolol, probably because vasodilatation led to higher peripheral blood flow with enhanced glucose uptake.18 Lipid profiles improved as well. These findings, if confirmed, could translate into a recommendation favoring carvedilol in patients with diabetes and CHF. Further information will emerge from the Carvedilol or Metoprolol Evaluation Trial, results of which will be available in about 2 years. Until then, any one of the 3 approved agents may be used.
Begin β-blocker therapy after euvolemia has been achieved through sodium restriction and diuresis and after adequate vasodilatation with ACE inhibitors or ARBs. Initiate therapy at a low dosage and increase it as tolerated with close follow-up, keeping in mind that the major trials used relatively high doses. In the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure, most patients received 200 mg/d of metoprolol (mean dose, 159 mg).15
Benefits may not be noted for weeks or even months following treatment initiation. Although a few patients may experience bradycardia or CHF decompensation, the discontinuation rate of β-blockers was typically lower than that of placebo in clinical trials.
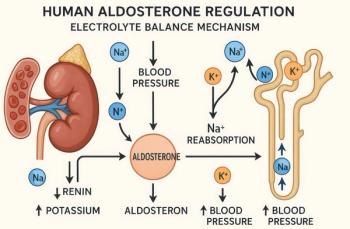
What is the role of aldosterone agonists in the treatment of CHF?
5The aldosterone antagonist spironolactone may be useful in carefully selected and closely monitored patients. In the Randomized Aldosterone Evaluation Study (RALES), spironolactone was added to the ACE inhibitor/β-blocker regimen of patients with New York Heart Association class III or class IV CHF. A 30% reduction in mortality was observed in this group.19 It should be noted, however, that patients with less severe heart failure were not part of the study and that relatively low doses of spironolactone were used. Moreover, patients were carefully followed for hyperkalemia, because both spironolactone and ACE inhibitors can raise serum potassium levels. An even greater risk of hyperkalemia exists when these agents are used together or when potassium supplements are used inappropriately. Unless proper precautions are taken, the excellent outcomes observed in the RALES cohort may not be reproducible.20,21
Are NSAIDs safe for patients with CHF?
6Although NSAIDs and cyclooxygenase (COX)-2 inhibitors are widely used for pain and inflammation by elderly persons, use of these agents should probably be discouraged in patients with CHF.
In these patients, renal function may depend on the activity of pros- taglandins, which are inhibited by NSAIDs.22 Inhibition of prostaglandin synthesis can result in fluid retention. In CHF patients, the use of NSAIDs may be implicated in cases of unexplained azotemia. NSAIDs may also interfere with the therapeutic properties of ACE inhibitors and loop diuretics, whose beneficial effects require the presence of local renal vasodilatory prostaglandins. Although COX-2 inhibitors have not yet been carefully studied in CHF patients, the above concerns could apply as well, because these agents have similar prosta- glandin-blocking properties.
In a case-control evaluation of elderly patients, NSAID use during the preceding week doubled the risk of hospitalization for CHF.23 A large prospective study did not implicate NSAIDs in new cases of CHF but did confirm their link with recurrent decompensation in patients with existing CHF.24 These considerations suggest that it may be best to advise patients with CHF to avoid using NSAIDs and COX-2 inhibitors.
How is diastolic heart failure best managed?
7Between 30% and 50% of patients with clinical CHF have normal systolic function of the left ventricle. Congestion is generally attributable to abnormal diastolic filling that may be a result of aging, hypertension, ventricular hypertrophy, or ischemic heart disease.25,26 Even during acute pulmonary edema, hypertensive CHF patients manifest intact or even supernormal systolic contractility.27
Diastolic CHF is often associated with concentric left ventricular hypertrophy of hypertensive origin. Pericardial constriction and infiltrative, obstructive hypertrophic, and restrictive cardiomyopathies are observed far less frequently. Ischemic heart disease is often present as well. Aging itself causes reduced myocardial and vascular compliance, with increased myocardial fibrosis and impaired relaxation in diastole. Clinical, ultrasound, nuclear, and hemodynamic methods can verify the diagnostic impression. Some researchers believe that more elderly women than elderly men are likely to have diastolic CHF.28
Diastolic CHF is best treated by volume control with diuretic therapy. Careful administration is required, because many patients with this condition have a narrow range of ideal central filling pressures (preload). Optimal regulation of systolic and diastolic blood pressure will minimize and perhaps even permit regression of ventricular hypertrophy, with subsequent improvement in diastolic filling.26,29 Because diastolic relaxation is partly an active energy-consuming process, myocardial ischemia should be treated vigorously, either pharmacologically or through surgical or transcatheter revascularization. A reduced heart rate will permit greater time for diastolic filling, and heart rate-lowering agents-such as a β-blocker, verapamil, or diltiazem-may act as direct myocardial relaxants as well.30 Because prospective controlled trials of diastolic CHF therapy have been limited in size and scope, treatment based on these general concepts is best tailored to each patient.31
References:
REFERENCES:1. Pitt B, Segal R, Martinez FA, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997;349:747-752.
2. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure. Randomised trial-the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355:1582-1587.
3. Cohn JN, Tognoni G, for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure (Val-HeFT). N Engl J Med. 2001;345:1667-1675.
4. Maggioni AP, Anand I, Gottlieb SO, et al, for the Valsartan Heart Failure Trial Investigators. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1420.
5. Packer M, Georghiade M, Young JB, et al, for the RADIANCE Study. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin converting enzyme inhibitors. N Engl J Med. 1993;329:1-7.
6. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525-533.
7. Pullicino PM, Halperin JL, Thompson JL. Stroke in patients with heart failure and reduced left ventricular ejection fraction. Neurology. 2000;54:288-294.
8. Dunkman WB, Johnson GR, Carson PE, et al, for the V-HeFT VA Cooperative Studies Group. Incidence of thromboembolic events in congestive heart failure. Circulation. 1993;87:94-101.
9. Al-Khadra AS, Salem DN, Rand WM, et al. Warfarin anticoagulation and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;31:749-753.
10. Bristow MR, Gilbert EM, Abraham WT, et al, for the MOCHA Investigators. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996;94:2807-2816.
11. Goldstein S, Kennedy HL, Hall C, et al. Metoprolol CR/XL in patients with heart failure: a pilot study examining the tolerability, safety, and effect on left ventricular ejection fraction. Am Heart J. 1999; 138:1158-1165.
12. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651-1658.
13. Colucci WS, Packer M, Bristow MR, et al, for the US Carvedilol Heart Failure Study Group. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. Circulation. 1996;94:2800-2806.
14. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9-13.
15. Effect of metoprolol CR/XL in chronic heart failure. Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
16. Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mor-tality, hospitalizations, and well-being in patients with heart failure. The Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000; 283:1295-1302.
17. Goldstein S. Benefits of beta-blocker therapy for heart failure. Weighing the evidence. Arch Intern Med. 2002;162:641-648.
18. Jacob S, Rett K, Wicklmayr M, et al. Differential effect of chronic treatment with two beta-blocking agents on insulin sensitivity: the carvedilol-metoprolol study. J Hypertens. 1996;14:489-494.
19. Pitt B, Zannad F, Remme WJ, et al, for the Randomized Aldactone Evaluation Study Investigators. The effects of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
20. Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211-214.
21. Tang WH, Francis GS. Spironolactone in chronic heart failure: all's well that ends well. J Am Coll Cardiol. 2003;41:215-216.
22. Gottlieb SS, Robinson S, Krichten CM, et al. Renal response to indomethacin in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;70:890-893.
23. Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med. 2000;160:777-784.
24. Feenstra J, Heerdink ER, Grobbee DE, Stricker BH. Association of nonsteroidal anti-inflammatory drugs with first occurrence of heart failure and with relapsing heart failure: the Rotterdam Study. Arch Intern Med. 2002;162:265-270.
25. Kitzman DW, Gardin JW, Gottdiener JS, et al, for the CHS Research Group. Importance of heart failure with preserved systolic function in patients > 65 years of age. Am J Cardiol. 2001;87:413-419.
26. Setaro JF. The hypertensive heart: new observations and evolving therapeutic imperatives. J Clin Hypertens (Greenwich). 2001;3:14-15.
27. Gandhi SK, Powers JC, Nomeir AM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17-22.
28. Masoudi FA, Havranek ED, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41: 217-223.
29. Warner JG, Metzger DC, Kitzman DW, et al. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol. 1999;33:1567-1572.
30. Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981-986.
31. Angeja BG, Grossman WG. Evaluation and management of diastolic heart failure. Circulation. 2003; 107:659-663.
FOR MORE INFORMATION:
- Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2001. Available at: www.acc.org/clinical/guidelines/ failure/hf_index.html. Accessed April 8, 2003.
- Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287: 628-640.
- Scaffidi R, Gottlieb SS. Heart failure at the beginning of the 21st century. Cardiovasc Rev Rep. 2001;22: 467-481.
Newsletter
Enhance your clinical practice with the Patient Care newsletter, offering the latest evidence-based guidelines, diagnostic insights, and treatment strategies for primary care physicians.