New Data Suggest neffy May be Safe, Effective for Treatment of Urticaria Flares
AAAAI 2024. In patients with treatment-resistant chronic spontaneous urticaria, neffy 1 mg and 2 mg resulted in improvement in itch, hives, urticaria, and erythema scores 5 minutes after dosing.
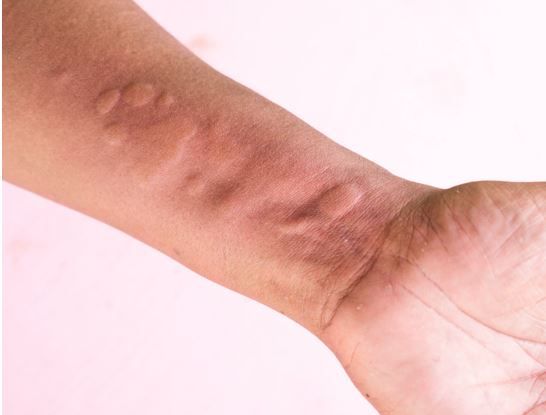
In a phase 2 study of patients with treatment-resistant chronic spontaneous urticaria (CSU), both 1 mg and 2 mg of an investigational epinephrine nasal spray (neffy®, ARS Pharmaceuticals) resulted in improvement in itch, hives, urticaria, and erythema scores as early as 5 minutes after dosing.1
Findings were presented today, February 26, 2024, in an oral presentation at the 2024 American Academy of Allergy, Asthma, and Immunology (AAAAI) annual meeting, February 23-26, 2024, in Washington, DC.
“The Phase 2 results in this highly refractory patient population are impressive and encouraging as they indicate neffy could be a game-changing therapeutic advance in the treatment of urticaria,” said presenting author David Bernstein, MD, emeritus professor of pediatrics, Cincinnati Children's Hospital Medical Center, in an ARS Pharmaceuticals press release. “Urticaria symptoms clear within minutes after dosing, and neffy could offer patients a novel treatment option to address existing gaps in the efficacy of currently available antihistamines or biologics.”2
According to researchers, symptoms of CSU are typically controlled with antihistamines or anti-IgE antibodies, however, many patients may still experience periodic acute exacerbation. A low-dose version of neffy™ (ARS-2) “is being developed as a needle-free option for the treatment of severe allergic reactions and is expected to be a highly effective, safe, and easy-to-use option for the rapid treatment of urticaria exacerbations,” they wrote in the abstract.1
Bernstein and colleagues conducted the study to evaluate the safety and efficacy of ARS-2 in patients with CSU treated with chronic medications who were still experiencing flares. The oral presentation given at AAAAI 2024 includes data from 18 adults as of the cut-off date who enrolled in the study and then returned to the clinical site while experiencing a flare, according to the press release.2
Participants were randomized to receive a single treatment of ARS-2 (1 mg and 2 mg) and placebo nasal spray “and then crossed over to other treatment during subsequent flare events,” stated investigators.1
Researchers assessed efficacy using patient-rated scores (patient-reported pruritus/hive score and visual analog scale for pain) and investigator-rated assessment (extent of urticaria and erythema score) from 5 minutes to 120 minutes after dosing.1
Results showed that both 1 mg and 2 mg doses of ARS-2 resulted in improvement beginning at 5 minutes and persisted for 120 minutes post-dose. There were no changes observed following the placebo spray, added authors.1
There were no meaningful differences in efficacy between the 2 doses of ARS-2. Investigators noted that statistically significantly improvements (P < .05) between ARS-2 and placebo were observed in all assessments.1
In addition, ARS-2 was well-tolerated, with mild-to-moderate adverse events reported in 8 participants, according to the press release. The most common adverse event was nasal discomfort in 5 participants; there were no serious adverse events reported.2
“This study adds to the wealth of data supporting the efficacy and safety of neffy in the treatment of type I allergic reactions,” said coauthor Sarina Tanimoto, MD, PhD, chief medical officer and cofounder of ARS Pharmaceuticals, in the release.2
Tanimoto continued: “Urticaria is not only a standalone type I allergy disease, but also represents the most frequent symptom observed during type I allergic reactions including anaphylaxis. The improvement in urticaria symptoms almost immediately after dosing neffy demonstrated its rapid onset of action, and is consistent with the previously reported responses on pharmacodynamic markers of efficacy in as little as one minute after dosing.”2
References:
- Bernstein D, Talreja N, Casale T, et al. ARS-2, low-dose intranasal epinephrine, improves urticaria scores in patients with frequent urticaria flares: Phase 2 study results. J Allergy Clin Immunol. 2024;153(2)(suppl):AB255. doi:10.1016/j.jaci.2023.11.817
- ARS Pharmaceuticals announces neffy® meets primary endpoints and shows rapid symptom control in Phase 2 urticaria clinical study. News release. ARS Pharma. February 26, 2024. Accessed February 26, 2024. https://ir.ars-pharma.com/news-releases/news-release-details/ars-pharmaceuticals-announces-neffyr-meets-primary-endpoints-and