Dupilumab in Replicate Phase 3 Trial Significantly Reduces COPD Exacerbations, Improves Lung Function, with PDUFA Date in Sight
Dupilumab reduced COPD exacerbations by 34% in adults with uncontrolled symptoms and evidence of T2 inflammation, and significantly improved lung function.
New data from the confirmatory phase 3 NOTUS trial of dupilumab used to treat chronic obstructive pulmonary disease (COPD) showed the versatile biologic significantly reduced the annualized rate of disease exacerbations by 34%, vs placebo, in study participants with uncontrolled symptoms and evidence of type 2 (T2) inflammation.1
Courtesy University of Alabama

The findings support the results of the 2023 replicate phase 3 BOREAS trial and enhance the potential for dupilumab to become the first new treatment approach for COPD in more than a decade and the first biologic agent indicated to treat the chronic lung disease.2
The NOTUS outcomes, presented at the 2024 American Thoracic Society International Conference and published simultaneously in the New England Journal of Medicine, were announced in a press statement on May 20 by codevelopers Sanofi and Regeneron.
“In my more than 20 years of practice, there have been limited advancements for patients struggling with the debilitating effects of uncontrolled COPD, and too many patients experience a vicious cycle of exacerbations that can result in loss of lung function and greatly diminish their quality of life.2
“In NOTUS, dupilumab reduced exacerbations by a magnitude never seen before with an investigational biologic in a phase 3 COPD clinical study. These comprehensive results reinforce that, if approved, dupilumab could provide a first-of-its-kind medical advancement for the COPD community,” coprincipal investigator Surya Bhatt, MD, MSPH, professor, division of pulmonary, allergy, and critical care medicine, at the University of Alabama at Birmingham, said in the press announcement.2
Dupilumab is under FDA priority review with a target action date currently set for June 27, 2024.
The NOTUS study evaluated use of dupilumab, a fully human monoclonal antibody that blocks interleukin-4 (IL-4) and IL-13 inhibitor, as add-on maintenance therapy in adults whose COPD symptoms were not controlled on maximal standard of care (SOC) therapy (the majority on triple therapy) and who had blood eosinophil count (BEC) levels of 300 cells/uL or greater, a key feature of T2 inflammation. All participants were either current or former cigarette smokers. Compared to those receiving placebo (n = 465) in the study, those receiving dupilumab (n = 470) experienced1:
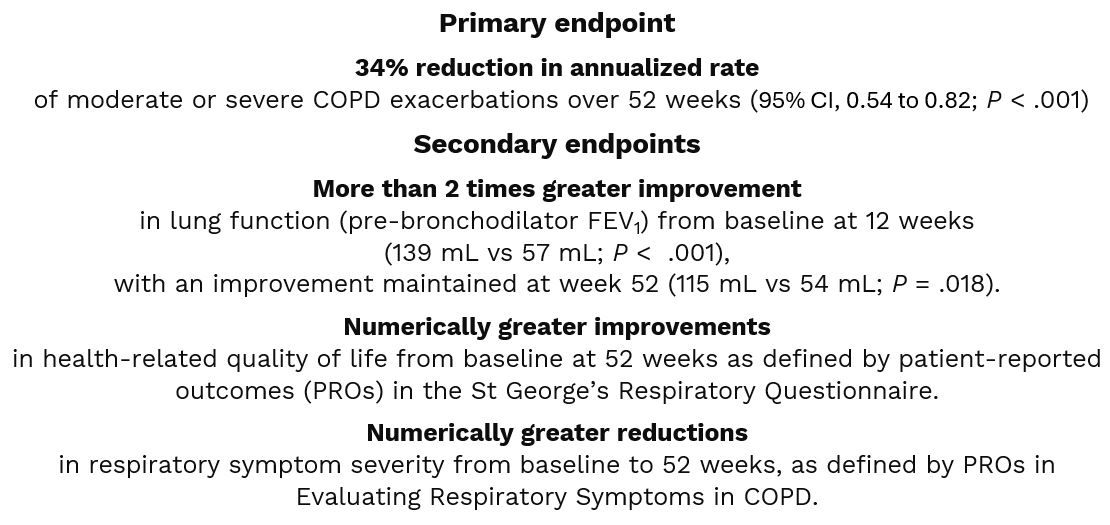
The findings mirror those from the BOREAS trial which demonstrated a 30% reduction in the annualized rate of moderate or severe COPD exacerbations in participants treated with dupilumab, results that Bhatt and colleagues elected to confirm in the nearly identical phase 3 NOTUS.1
The 1874 participants across the 2 studies (approximately 67% men) ranged in age from 40 to 85 years (mean age 65 years) and received dupilumab or placebo every 2 weeks on background therapy of maximal SOC consisting of inhaled corticosteroids (ICS), long-acting beta agonists (LABA), and long-acting muscarinic antagonists (LAMA) of a stable dose for at least 1 month. Double maintenance therapy, which included LABA and LAMA, was allowed if ICS was contraindicated. All participants in the year prior to screening must have had at least 2 moderate or 1 severe COPD exacerbation, according to the study.1
Investigators reported that participants in both studies who received dupilumab experienced rapid and significant improvements in lung function by as early as 12 weeks, gains that were sustained at 52 weeks.
Bhatt et al also found that reductions in the frequency of exacerbations over the 52-week study were similar across the prespecified subgroups defined by age, sex, smoking status, lung function at baseline, exacerbations history, and absence of emphysema.1
Among the study limitations the authors note the homogeneity of the population and that the trial was conducted during the global COVID-19 pandemic, the latter potentially introducing a range of unidentified confounders that could limit generalizability of the results. Also, reduced sample size related to early primary analysis reduced the statistical power of some of the 52-week endpoints.
Nonetheless, the team concluded: “These results further confirm the role of type 2 inflammation in the pathobiologic disease mechanisms in a subgroup of patients with COPD and the role of dupilumab in treating this distinct COPD endotype.”1
References
1. Bhatt SP, Rabe KF, Hanania NA, et al, for the NOTUS Study Investigators. Dupilumab for COPD with blood eosinophil evidence of type 2 inflammation. N Engl J Med. Published May 20, 2024. doi:10.1056/NEJMoa2401304
2. Dupixent late-breaking data from NOTUS confirmatory phase 3 COPD study presented at ATS and published in NEJM. News release. Sanofi. May 20, 2024. Accessed May 20, 2024. https://www.news.sanofi.us/2024-05-20-Dupixent-R-late-breaking-data-from-NOTUS-confirmatory-phase-3-COPD-study-presented-at-ATS-and-published-in-NEJM
Newsletter
Enhance your clinical practice with the Patient Care newsletter, offering the latest evidence-based guidelines, diagnostic insights, and treatment strategies for primary care physicians.