Treatments with the investigational rescue medication achieved clinically effective epinephrine levels faster than intramuscular injection.

Treatments with the investigational rescue medication achieved clinically effective epinephrine levels faster than intramuscular injection.
The updated guidelines include 19 recommendations related to the diagnosis and management of eosinophilic esophagitis.

Aquestive Therapeutics announced positive feedback from the FDA this week that keeps NDA planning for the novel epinephrine product on solid ground.
The target action date for the FDA decision is April 18, 2025, according to Sanofi and Regeneron.

A decision on ending use of oral phenylephrine as a nasal decongestant in OTC products will be made following public comment on FDA's proposed order.
ACAAI 2024. Two studies here showed that anaphylaxis protocols for EMS are outdated in many states and that many patients don't know how to use epinephrine.

The company is advancing development of the intranasal epinephrine medication following positive phase 1 data.

A summary of new data presented at the 2024 American College of Allergy, Asthma & Immunology Annual Scientific Meeting.

Updated mRNA COVID-19 vaccines, the first orally disintegrating tablet approved for pregnancy prevention, and 5 more.

The needle-free nasal spray, if approved, would be the first new administration method for epinephrine in young children in 35 years.

Your daily dose of the clinical news you may have missed.

The approval of neffy (epinephrine nasal spray) could help individuals and caregivers act sooner and feel safer treating a type 1 allergic reaction.

neffy (epinephrine nasal spray) is the first alternative to epinephrine administered by autoinjector or syringe and is comparable in efficacy to the gold standard.

PK data from a self-administration study are comparable to those seen following administration by a clinician and Tmax equal at 15 minutes.

Positive findings from a repeat dosing study of neffy under nasal allergen challenge conditions will support a potential PDUFA date of October 2, 2024, the company said.
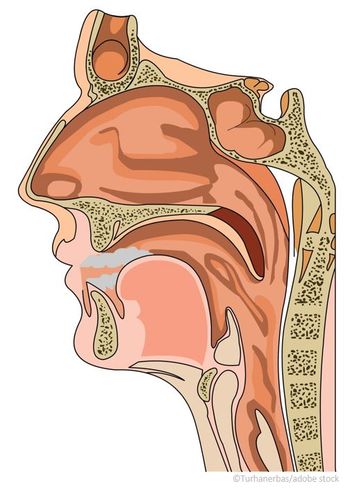
Now FDA approved, Optinose’s Xhance is the first nonsurgical option for chronic rhinosinusitis both with and without nasal polyps.

Your daily dose of the clinical news you may have missed.

Dr Ebisawa details the findings he recently presented at the 2024 AAAAI annual meeting, including adverse events and next steps for the study.

AAAAI 2024. Needle phobia is just one of the barriers to not just using an epinephrine autoinjector but to carrying one at all, according to 2 sets of surveys findings.

AAAAI 2024. In patients with treatment-resistant chronic spontaneous urticaria, neffy 1 mg and 2 mg resulted in improvement in itch, hives, urticaria, and erythema scores 5 minutes after dosing.

neffy (epinephrine nasal spray) delivered a PK and PD profile greater than or similar to that of IM epinephrine after extreme nasal challenge, reports ARS Pharmaceuticals.

AAAAI 2024. Dupilumab-treated eosinophilic esophagitis in children was also more often associated with comorbid asthma, according to researchers.

AAAAI 2024. New data on neffy (epinephrine nasal spray) include the first report of efficacy after oral food challenge in a pediatric population at risk for anaphylaxis.

AAAAI 2024. Reduction of AD signs and symptoms was significant among approximately half of study participants at 4 weeks and continuously rose through week 260, investigators report.

AAAAI 2024. Oral mucosal immunotherapy delivered via metered-dose toothpaste produced a statistically significant increase in IgG4 and a decrease in IgE/IgG4 ratio.