
An experimental cell-based therapy induces permanent allergen-specific immunologic tolerance to prevent IgE-mediated allergy.

An experimental cell-based therapy induces permanent allergen-specific immunologic tolerance to prevent IgE-mediated allergy.

Why has the number of food allergies increased but overall sensitization to food allergens remained static?

Spring has sprung! Click here for the latest research about some of the more common diseases of the season.

Results of a new study help explain why your adult patients with atopy most likely have autumn birthdays.

Poor agreement between caregiver and children for report of symptoms and relief presents a challenge in clinical trials.
The emerging therapy may help overcome drawbacks of existing immunotherapies, such as treatment duration and poor adherence.

Hay fever is common in adults age 65 years and older but there is little research on the efficacy of customary immunotherapy in this group.

The once-daily Oralair tablet may considerably change the experience of seasonal allergy sufferers.

Current and emerging treatment options for ragweed-induced rhinoconjunctivitis are the topic of a comprehensive review paper--a good read for all primary care providers.

The discovery of decreased bioavailability of the anithistamine fexofenadine after ingestion of grapefruit juice broadened understanding of mechanisms underlying these sometimes deadly drug-juice interactions.
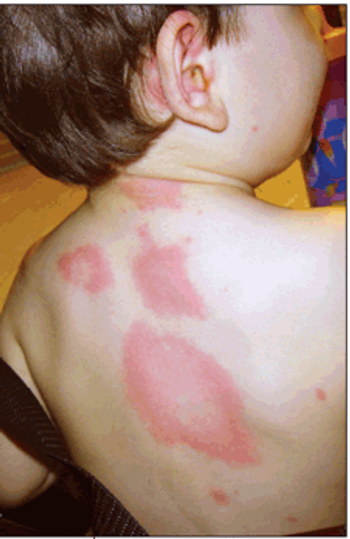
Allergic rhinitis and the “allergic triad,” allergy tests for children, allergic asthma, acute urticaria, a lipstick allergy, allergic contact dermatitis of the scalp-these images show a range of common allergic conditions.
Spring is here and brings with it the peak allergy season. Patients will present with a variety of allergic diseases, both seasonal and otherwise. This week’s photo quiz tests your knowledge of these disorders.
Diabetes progress; folic acid and autism; acupuncture and allergic rhinitis; depression and shingles vaccine; HMP virus alert.

Acupuncture for patients with seasonal allergic rhinitis was found to be more effective than antihistamine use or a sham intervention.

To allay children's fears about immunizations or allergy shots, keep a pocket-sized, battery-operated radio in the office.

Physical clues to allergic rhinitis include the allergic crease on the dorsum of the nose and allergic "shiners" or periorbital ecchymosis.

For my patients who use nasal spray, I advise them to use the right hand to spray into the left nostril and the left hand to spray into the right nostril.

Many pharmacological options exist for allergic rhinitis. Intranasal corticosteroids are the most effective medication class for patients with moderate to severe symptoms; those with milder intermittent symptoms can be treated with a second-generation oral or intranasal antihistamine.

A 14-year-old girl is seen because of long-standing nasal itch, intermittent nasal congestion, and clear nasal discharge. Allergies to house dust mites and to cats shown on prior skin testing. Often has marked eye swelling when exposed to cats. Receives antihistamines when in flare-up and prior to cat exposure. The consumption of dairy products exacerbates all of the above.

While avoidance measures are a key component of the treatment of allergic rhinitis, pharmacological therapies are often needed to adequately control symptoms. Intranasal corticosteroids are highly effective and are particularly useful in patients with moderate to severe disease.

Advise patients who are receiving continuous positive airway pressure (CPAP) therapy and who use a corticosteroid nasal spray for allergies to spray in the morning rather than at bedtime. If the spray is used in the evening, CPAP may dry it out, making it less effective.

Allergic rhinitis is highly prevalent; about 20% of adults in the United States and 25% of children worldwide are affected. It is a major societal expense, with direct costs, attributable to physician visits and medications, of up to $5 billion per year, and indirect costs, mainly stemming from lost productivity, of up to $9.7 billion per year. In the United States, allergic rhinitis results in 3.5 million lost workdays and 2 million lost schooldays each year.
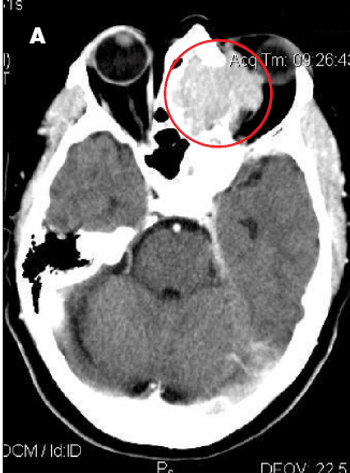
Progressively worsening nasal congestion and headaches with diplopia and left proptosis for 2 months prompted an ophthalmology consultation for a 67-year-old woman. She had been evaluated multiple times for allergic rhinitis and recurrent sinusitis.
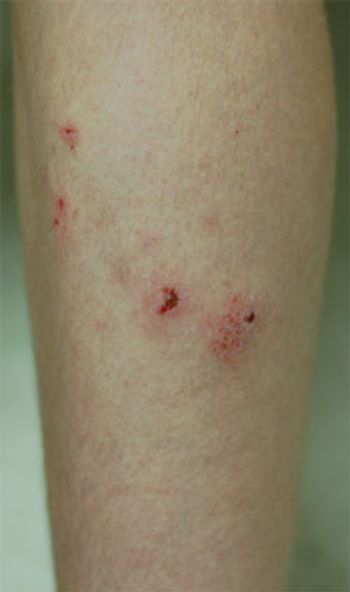
For the past year, a 15-year-old boy has had a pruritic eruption on his shins. His mother suspects that his soccer shin guards are the cause; however, he wears them over his socks. Topical corticosteroids have not been effective.

A 10-year-old girl with seasonal allergies has had this pruritic patch on her arm for a few days. She denies any exposure history.