
NIH researchers also proposed new diagnostic criteria that include a combination of major and minor symptoms, offering improved sensitivity for identifying TSW cases.


NIH researchers also proposed new diagnostic criteria that include a combination of major and minor symptoms, offering improved sensitivity for identifying TSW cases.

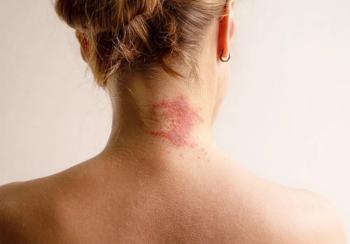
Antihistamines for itch caused by disorders like atopic dermatitis pose more problems, like sedation and fall risk, than they provide relief, according to Daniel Butler, MD.

Both trials met their primary endpoints, demonstrating a statistically significant improvement in HiSCR50 compared to placebo, with a favorable safety profile, according to Incyte.

Elizabeth Swanson, MD, shares how the rise of T.indotineae, a terbinafine-resistant fungal species, has shifted her treatment approach.

AAD 2025: Tracing the multiple pathways that contribute to chronic itch vs looking for a singular cause helps optimize choice of therapy, Butler says.

Shingles is increasingly being observed in healthy, immunocompetent children. Elizabeth Swanson, MD, discusses how to recognize and respond.

Dr Swanson discusses the resurgence of measles and emphasizes the safety and efficacy of the measles vaccine.
Dupilumab-treated participants with recalcitrant head and neck symptoms who were switched to upadacitinib rapidly reached EASI 75 and steadily improved, among other findings.

Elizabeth Swanson, MD, discusses the changing face of HFMD, focusing on the increasingly prevalent coxsackievirus A6 strain and its unique characteristics

In an interview at AAD 2025, Dr Yu emphasized the importance of managing contact dermatitis early and effectively to prevent long-term complications.

The impact of childhood onset AD on adult psychosocial experience, including employment and the decision to become a parent, is profound, this study reveals.

The findings highlight the potential of the interleukin-23 inhibitor to address a notoriously difficult-to-treat manifestation of psoriasis.

There are 3 classes of nonsteroidal topical therapies for atopic dermatitis that target the specific underlying pathophysiology of the skin disease. Shahriari provides a primer as physicians prepare for AAD 2025.

The company announced completion of target enrollment in the REZOLVE-AA study of rezpegaldesleukin in people with severe-to-very-severe alopecia areata.

Investigators reported that roflumilast cream 0.05% significantly reduced AD severity as early as Week 1, with a notable effect on pruritus seen in as little as 24 hours.

Treatment of AD with dupilumab, compared with cyclosporine and methotrexate, was associated with significantly reduced risk of a wide range of CV outcomes at 1 year.

The clearance enables Alphyn to initiate global phase 2b clinical trials, which are anticipated to begin in the first quarter of 2025.

Rezpegaldesleukin targets the IL-2 receptor complex to stimulate regulatory T cells, aiming to restore immune balance in people with atopic dermatitis.

The investigational molecule with “JAK-inhibitor-like efficacy and IL4/IL13-like safety,” was cleared in Q4 2024 by the FDA for an IND application, the company said.

Your daily dose of the clinical news you may have missed.

With no FDA-approved treatments for moderate-to-severe chronic hand eczema in the teen population, the late-stage topline data are very promising.

An oral antibiotic for drug-resistant UTIs, a long-awaited therapy for congenital adrenal hyperplasia, and more.

Tapinarof 1% cream when used as topical monotherapy led to statistically significant and sustained improvements in QoL, sleep, and other PROs across age groups.

There are no diagnostic criteria for topical steroid withdrawal but patients describe and discuss it on social media. Study authors tapped a deep source of information.

The key factors predicting stable response to tralokinumab every 4 weeks (vs every 2 weeks) were IGA 0/1 and EASI-75, according to a small post hoc analysis.