
Dupilumab shows superior 2-year drug survival rates in atopic dermatitis compared to other biologics and JAK inhibitors, highlighting its effectiveness in treatment persistence.

Dupilumab shows superior 2-year drug survival rates in atopic dermatitis compared to other biologics and JAK inhibitors, highlighting its effectiveness in treatment persistence.

Two global phase 3 trials found rocatinlimab significantly improved EASI-75 and vIGA-AD outcomes at 24 weeks in people with moderate-to-severe AD with acceptable safety.

KT-621 achieved deep STAT6 degradation and strong 4-week EASI and itch reductions, offering a potential new oral option for moderate–severe AD and other Th2 inflammation-driven disease.

Psychiatric comorbidities affect 25% of AD patients. Here, a check list on when to screen, when to refer, and how to prevent accumulated psychological burden in primary care.

A regular focus on mental heatlh during follow-ups with patients who have atopic dermatitis can be integrated simply with a few thoughtful questions.
Controlling atopic dermatitis signs and symptoms early can alter social, emotional, and functional outcomes, sometimes dramatically.

Emotional red flags in AD, eg, sleep loss, isolation, hopelessness, often go unseen. Quick screening questions can surface the real burden individuals carry.

Growing up with AD carries lifelong emotional consequences. Clinicians who recognize these developmental risks can better support patients at every stage.

Up to 1 in 4 patients with atopic dermatitis face anxiety or depression. Primary care clinicians are key to identifying risk and supporting mental well-being.

LEO Pharma reveals promising 32-week results for tralokinumab, showing significant benefits for adults with moderate-to-severe hand eczema.

ACAAI 2025. Phase 3 data show tapinarof cream provides early, sustained improvement and good tolerability in children aged ≥2 years with atopic dermatitis.

Phase 2b findings show rezpegaldesleukin reduced ACQ-5 scores and improved key AD endpoints in participants with comorbid asthma.

The approval of roflumilast 0.05% provides a steroid-free, once-daily treatment option for safe and effective long-term management of pediatric eczema.

EADV 2025: Guttman-Yassky has great confidence in the future of agents that block the OX40 pathway, suggesting that they have the potential for disease modification.

Incyte announced the agency's decision, which was based on findings of significant reduction in atopic dermatitis signs and symptoms during the TRuE-AD3 clinical trial.

EADV 2025: Guttman-Yassky presented data from the phase 3 ROCKET-IGNITE trial and highlights its importance in the context of the global ROCKET development program.

EADV 2025: Phase 3 ROCKET-SHUTTLE results showed the T-cell rebalancing therapy significantly improved AD signs and symptoms in treatment-experienced adults.

The novel IL-22 blocker was associated with improvements in EASI as early as week 1 for lower doses and week 2 for the highest dose, compared with placebo.

EADV 2025: New phase 3 data reveal delgocitinib cream's efficacy for chronic hand eczema in adolescents, confirming its safety profile in adults.
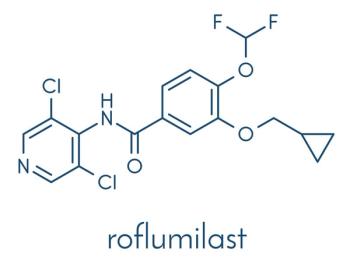
EADV 2025: Data will highlight the PDE4 inhibitor's efficacy across diverse populations and 3 common skin conditions: seborrheic dermatitis, atopic dermatitis, and psoriasis.
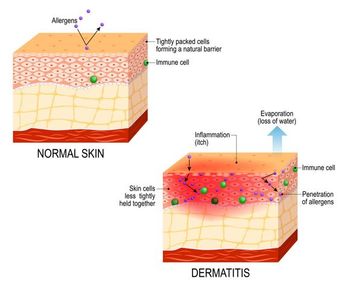
MRGPRX2 antagonism is the only known mechanism of action designed to modulate mast cells and sensory neurons, both key drivers of lesions and itch in AD.

Discover the latest advancements in atopic dermatitis treatment, including new topical agents and biologics, enhancing care for patients with moderate to severe conditions.

Linda Stein Gold, MD, reviews AAD’s 2025 updates on new topical and biologic options for adult atopic dermatitis.

Insights on atopic dermatitis treatment preferences, the safety of childhood vaccines, maternal depression’s effect on parenting, and more.

Your daily dose of the clinical news you may have missed.