
How does alcohol figure in to American holiday celebrations? We checked with Alcohol.org for this quiz. Are answers what you expect?











How does alcohol figure in to American holiday celebrations? We checked with Alcohol.org for this quiz. Are answers what you expect?

Episode highlights include a new AD blood test for PCPs, sleep effects of elinzanetant among postmenopausal women, lung cancer screening gains, and more.

Ob/gyn Dorr outlines how short clinical visits and symptom overlap cause many women to receive an antidepressant Rx instead of perimenopause care.

Symptoms of the menopausal transition can be so disruptive that women consider leaving employment and yet many report misdiagnosis and inappropriate treatment.

Highlights include widespread lack of structured menopause education in primary care residency programs, sleep benefits of elinzanetant, and more.

Your daily dose of the clinical news you may have missed.

A recent meta-analysis reveals that two-thirds of pregnancies experience unhealthy weight gain, highlighting risks for mothers and babies.

Dorr says symptoms can begin 10 years before cylces cease and explains why the hormonal feast and famine of perimenopause can go unrecognized.

Lisa Larkin, MD, discusses how social media is driving menopause awareness and why primary care physicians must educate themselves to meet patient demand.

Cochrane shares her own research findings on how clinician specialty shapes menopause care and highlights gaps in hormone therapy prescribing.

Larkin Larkin, MD, reviews treatment options for VMS, comparing hormone therapy effectiveness with antidepressants and new neurokinin antagonists.

TMS: Harvard's Caroline Mitchell, MD, MPH, questions the direction of genitourinary syndrome research priorities and reflects on FDA's removal of HRT warnings.

Women increasingly seek evidence-based menopause care as awareness grows, yet many still suffer from untreated vasomotor symptoms.

Harvard's Caroline Mitchell, MD, MPH, explains how common OTC products can worsen vulvovaginal symptoms and the importance of a targeted history in GSM care.

Donna Plecha, MD, discusses how AI-powered mammography risk prediction and polygenic risk scores will enable personalized breast cancer screening within the next decade.

Breast imaging expert, Donna Plecha, MD, provides a roadmap for primary care physicians to implement risk assessment tools and high-risk clinic referrals in their practice.
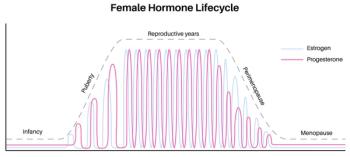
Nearly 40% of perimenopausal women report misdiagnosis as clinicians treat for anxiety and depression without addressing underlying hormonal imbalance.

New research links high ultra-processed food intake to increased risk of precursors for early-onset colorectal cancer in women under 50.

The comprehensive timeline highlights how WHI findings and subsequent research reshaped HRT risk assessment, guidance, and FDA labeling through 2025.

Access, cost, and clinician awareness remain significant barriers to implementing supplemental breast screening, according to Plecha.

Breast imaging expert Donna M. Plecha, MD, explains the FDA's dense breast notification requirement and when to recommend supplemental MRI screening.

Mitchell highlights hormone therapy prescribing for primary care: treatment algorithms, vaginal estrogen safety, and when progestins aren't needed.

TMS 2025: Katrina Wugalter, MA, discusses research showing that age—not menopause stage—drives brain volume decline, and how lifestyle factors can help preserve brain health in midlife women.

Regulatory update aims to align labeling with current evidence on menopausal hormone therapy safety and benefits

TMS 2025: Dr Marla Shapiro doesn't start perimenopausal therapy without an answer to the most important question: "What is your most bothersome symptom?"