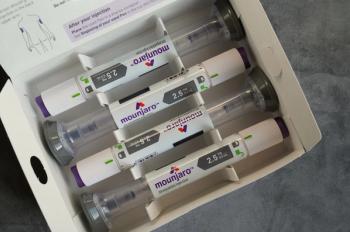
Update: The FDA has told compounding pharmacies they may resume production of tirzepatide products while the agency further investigates supply.

Update: The FDA has told compounding pharmacies they may resume production of tirzepatide products while the agency further investigates supply.
Proposed indications for the investigational factor XIa inhibitor are: stroke prevention after acute ischemic stroke or high-risk transient ischemic attack; recent acute coronary syndrome; and atrial fibrillation.
Published: June 7th 2023 | Updated: