
The CDC website now claims "studies supporting a link [between vaccines and autism] have been ignored by health authorities."

The CDC website now claims "studies supporting a link [between vaccines and autism] have been ignored by health authorities."

Upadacitinib (15 and 30 mg) was evaluated in adolescents with moderate to severe AD aged 12 to 17 years, with and without topical corticosteroids.
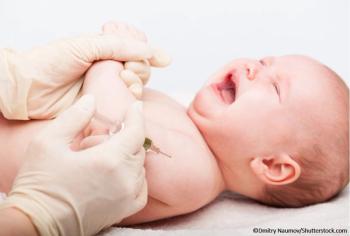
The ACIP was unanimous in its recommendation for routine use of nirsevimab-alip to prevent RSV LRTD in newborns and young infants during their first RSV season.

FDA approval of the monoclonal antibody against RSV follows the unanimous vote from the agency's Antimicrobial Drugs Advisory Committee.
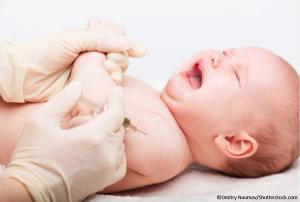
Published: July 17th 2023 | Updated:

Published: August 3rd 2023 | Updated: