Biosimilars Wyost and Jubobonti were approved as interchangeable for Xgeva and Prolia but launch will depend on the outcome of litigation between reference product manufacturers.

Biosimilars Wyost and Jubobonti were approved as interchangeable for Xgeva and Prolia but launch will depend on the outcome of litigation between reference product manufacturers.

FDA's action, based on confirmatory evidence showing slowed disease progression, follows a January 2023 accelerated approval.

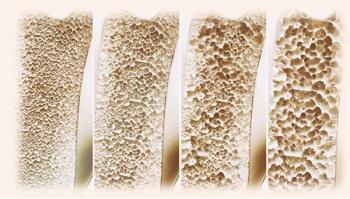
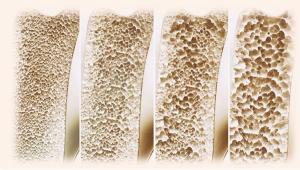
Results of a new study offer additional support for clinical guidelines to favor one therapy over another, authors suggest.
Published: March 6th 2024 | Updated:

Published: July 7th 2023 | Updated:

Published: June 12th 2023 | Updated: