
Nuvaxovid (NVX-CoV2373) is indicated for those aged ≥65 years and aged 12 to 64 years with at least one condition that puts them at high risk for severe infection.

Moderna's mRNA-1010 Flu Vaccine Proves Superior Efficacy in Pivotal Late-Stage Trial

Nuvaxovid (NVX-CoV2373) is indicated for those aged ≥65 years and aged 12 to 64 years with at least one condition that puts them at high risk for severe infection.

Cases of whooping cough in the US more than doubled in 2024, and 2 infants have died from the infection. Here is background on the infection and vaccine.

Misinformation about measles and the MMR vaccine has reached the majority of US adults and parents; most believe the vaccine is safe but many are still unconvinced.
Novavax’s COVID-19 vaccine was linked to fewer and less severe side effects vs mRNA in a University of Utah study of health care workers.

Respiratory syncytial virus was deadly and untreatable across the lifespan until 2023 when the first vaccines were approved in the US. Follow the timeline, here.
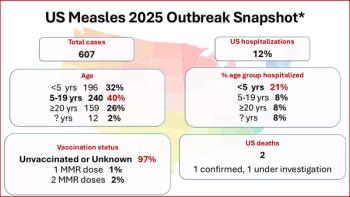
As the US measles outbreak tops 600 cases, Texas reported a second child's death in a young girl who was unvaccinated and had no underlying health conditions.
The FDA granted fast track designation to Sanofi’s chlamydia vaccine candidate today, aiming to prevent infections and address an unmet public health need.
CROI 2025: Increased HPV vaccine coverage could reduce HIV cases by thousands, according to a new study.

Get the latest immunization updates, data on racial disparities, and practical guidance for clinical practice, here.

Your daily dose of the clinical news you may have missed.

The Metrix COVID/Flu molecular test detects and differentiates SARS-CoV-2, influenza A, and influenza B in approximately 20 minutes.

The ACIP of the CDC will meet on February 26 to vote on recommendations for appropriate incorporation of Penmenvy into the routine vaccination schedule.

Cardiovascular comorbidities in adults with COPD are linked to poor outcomes including reduced QoL, increased hospitalizations, and greater risk of mortality, Han explains.

People with chronic diseases like COPD or with immunocompromising conditions continue to have reservations about vaccines against flu, COVID-19, and RSV.
The novel therapeutic demonstrated a strong safety profile and showed efficacy in reducing symptoms during the early stages of treatment.

Guidance from the Advisory Committee on Immunization Practices on best practices for COVID-19 vaccination continues to evolve, Hopkins explained.
The FDA approved the addition of Guillain-Barré syndrome warnings for Abrysvo and Arexvy, citing postmarketing data suggesting an increased risk.

Robert Hopkins, Jr, MD, NFID medical director, details a range of resources on the foundation's website including in-depth vaccine recommendations, live webinars, plus much more.

Under risk-based guidelines, less than 30% of eligible adults are vaccinated against pneumococcal disease, said Robert Hopkins, Jr, MD. More details, here.
The addition, based on an approved update to labeling, expands to 4 the recommended vaccination options that health care professionals can offer to pregnant people.
The 2 novel vaccine candidates each combine a Sanofi licensed influenza vaccine with the Novavax adjuvanted recombinant COVID-19 vaccine.

Your daily dose of the clinical news you may have missed.

Your daily dose of the clinical news you may have missed.

Little action has been taken by state medical boards against clinicians who spread unsubstantiated claims about COVID-19, vaccines, or other pandemic-related topics.
The FDA cleared Novavax to start enrolling the planned phase 3 trial after a safety concern was found to be unrelated to the combination vaccine.