Oral estrogen hormone therapy was more likely to result in incident hypertension than transdermal or vaginal formulations in this large prospective population-based study.

Oral estrogen hormone therapy was more likely to result in incident hypertension than transdermal or vaginal formulations in this large prospective population-based study.
Menstrual migraine is often described as more disabling than non-menstrual migraine. Get an overview and test your knowledge, here.

Your daily dose of clinical news you may have missed.

A nationally recognized obesity expert discusses mental health in women with obesity.
The findings contradict published clinical guidelines that warn against use of estrogen-containing birth control by women who have migraine with aura.

Your daily dose of clinical news you may have missed.

Women's Health Week (May 14-20, 2023) is an ideal opportunity to review the top research, policy shifts, and potential drug approvals that affect your patient panel.

Fezolinetant is the first NK3 receptor antagonist approved by the FDA for treatment of moderate-to-severe vasomotor symptoms due to menopause.

Your daily dose of clinical news you may have missed.

Opill, a progestin-only oral contraceptive, if approved by the FDA, would be the first an only form of birth control in the US available without a physician's prescription.
Denosumab reduced RR of fracture vs alendronate across fracture types, eg, by 36% for hip fracture, by 43% for nonvertebral fractures, and by 30% for vertebral fractures.
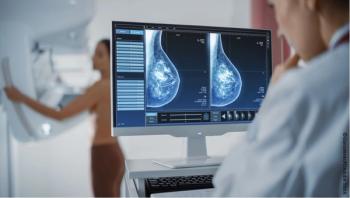
The lower recommended starting age for screening reflects rising cancer diagnoses among younger women and persistently high mortality rates among Black women.
Results of a Mendelian randomization study showed earlier first birth, higher number of live births, and earlier menarche are all associated with increased CVD.

Sex-based disparities found in high-profile clinical trials presented at 3 key cardiology scientific meetings underscore the lack of progress made toward equitable inclusion in research.

Tirzepatide delivers 15.7% weight loss in adults with obesity; remote intervention increases cancer screening in women; women at greater post-MI risk vs men; plus 2 more updates of note.

Your daily dose of clinical news you may have missed.

Young women who survived a myocardial infarction may have nearly double the risk of rehospitalization in the year after discharge compared to men, according to a new study.

Your daily dose of clinical news you may have missed.

The updated American College of Physicians osteoporosis treatment guideline is summarized in this topline review for primary care clinicians.
Infants with neonatal opioid withdrawal syndrome cared for using a novel functional approach were discharged nearly 7 days sooner than those treated with usual care.

A combination of outreach measures tailored to the needs of rural Midwest women increased recommended screenings by as much as 6-fold.

Women with prior HC-associated depression had a higher risk of developing a depressive episode during pregnancy and postpartum than women with non-HC-associated depression.

Caroline Apovian, MD, highlights how drugs like semaglutide 2.4 mg treat the chronic relapsing disease of obesity and why a distinction from "weight-loss drugs" is critical.

Race- and ethnicity-specific starting ages for screening would help prevent early breast cancer mortality among Black women, according to authors of a new study.

Statins in AF patients cut risk of TIA; opioid makers must strengthen label warnings; individualized Rx reduces BP; and 2 more research quotes of note.