
Mepolizumab, an IL-5 inhibitor targeting Th2 inflammation characterized by elevate serum eosinophil count, fills a treatment gap for ~70% of adults with uncontrolled COPD.

Mepolizumab, an IL-5 inhibitor targeting Th2 inflammation characterized by elevate serum eosinophil count, fills a treatment gap for ~70% of adults with uncontrolled COPD.

Your daily dose of the clinical news you may have missed.

At ACOG 2025, Elizabeth Mollard, PhD, discussed her research on Medicaid PPD screening and coverage policies on PPD diagnosis.

Itch relief in clinical trials was rapid with the nonsteroidal foam; it is applied once daily with no limitations on body area or duration of use.

Results showed that a nanoencapsulated CBD cream reduced UV-A–induced nuclear and mitochondrial DNA damage linked to photoaging in human skin.

Moderna voluntarily withdrew the BLA after consultation with the FDA and will resubmit once it has additional phase 3 efficacy data for its investigational flu shot.

The WAYFINDER trial is the first to demonstrate the positive impact of the thymic stromal lymphopoietin antagonist on steroid-dependent severe asthma.

Your daily dose of the clinical news you may have missed.

ACOG 2025: Laxmi Gannu, MS, discusses key findings from a provider survey on postpartum depression screening practices, training gaps, and referral patterns.

ACOG 2025: Joy Baker, MD, urged primary care clinicians to screen early, refer confidently, and help change the statistics on maternal mental health.

Data from the global RAPID registry provide real-world support for dupilumab efficacy in youths and adults with uncontrolled asthma and with or without CRS and/or nasal polyposis.

ACOG 2025: Johanna Finkle, MD, shares strategies to start weight conversations with patients and build clinician confidence in counseling and treatment options.
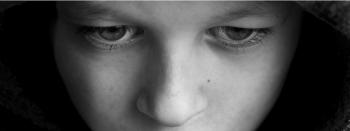
Youth depression is often diagnosed during crisis events, underscoring the need for primary care education and enhanced collaborative care resources.

The FDA's new approach to evaluating and approving COVID-19 vaccines will move the US away from a one-size-fits-all approach and closer to policy in other countries.

Your daily dose of the clinical news you may have missed.

ACOG 2025: Elizabeth Mollard, PhD, discusses her research on how Medicaid policies influence postpartum depression screening rates.

Your daily dose of the clinical news you may have missed.

Adults with early AD who were treated with lecanemab in a specialty memory clinic experienced adverse events comparable to those seen in clinical trials.

ACOG 2025: The Junonia study was designed to understand gaps in care for women with PPD using surveys of their clinicians and care coordinators.

ACOG 2025: Joy Baker, MD, discusses the importance of listening closely to how patients describe postpartum distress—and to read between the lines.