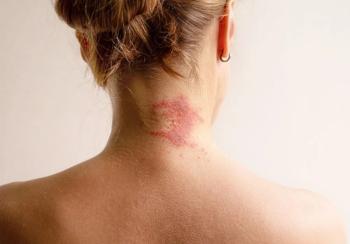
Adults receiving the highest dose achieved 2 times the reduction in EASI scores vs placebo with responses emerging as early as day 8 of treatment.

Adults receiving the highest dose achieved 2 times the reduction in EASI scores vs placebo with responses emerging as early as day 8 of treatment.

The ability to predict which individuals will experience adverse events from GLP-1 therapy has great promise for more personalized treatment and improved clinical trial design.

Your daily dose of the clinical news you may have missed.

The IL-22FA1 antagonist achieved positive outcomes for the primary endpoint based on percentage change in EASI from baseline to week 16 for 3 highest doses, according to LEO.

Data for GC reduction and improved A4 levels were consistent across subgroups stratified by sex, race, age, BMI, and baseline A4 levels, according to Neurocrine Biosciences.

An interview with Jeff Andrews, MD, FRCSC, of BD Life Sciences.

The investigational mRNA-1083 vaccine induced superior immune responses against all 4 flu strains in adults aged 50-64 years and against 3 in adults 65 and older.

Your daily dose of the clinical news you may have missed.

The chronotherapeutic approach aligns treatment with peak glucocorticoid sensitivity, so may mitigate onset of the inflammatory cascade that triggers nocturnal exacerbations.

Any increase in lipoprotein(a) levels was linked to increased risk of myocardial infarction and stroke; women and Black adults are most likely to be affected.

Low-dose clorthalidone offers clinicians a new option for initiating treatment that may mitigate well-known metabolic adverse effects.

Your daily dose of the clinical news you may have missed.

Publication of the phase 3 ARRECTOR trial findings precede an anticipated PDUFA date of May 22, 2025, for the investigational foam, now under FDA review.

PODCAST: Listen to our first episode for new research on bacterial vaginosis, a new treatment option for chronic urticaria, and more.

The model demonstrated 70.63% accuracy for predicting depression, a common comorbidity seen in COPD and one that increases risk for negative outcomes.

ZORYVE® (roflumilast) cream 0.15% is a once-daily, steroid-free PDE4 inhibitor approved for the treatment of mild to moderate atopic dermatitis in patients aged 6 and older. Clinical trials demonstrate its rapid onset, strong efficacy, and favorable safety profile, with emerging evidence supporting its use in younger children and as a long-term maintenance therapy to prevent flares.

EASL 2025. DA-1241 significantly reduced plasma ALT levels, improved systemic inflammatory and fibrosis biomarkers, according to drug developer MetaVia.

An interview with Jeff Andrews, MD, FRCSC, of BD Life Sciences.

Primary care clinicians are on the frontline of detecting early cognitive decline. Help us understand your challenges by taking this short survey.