
Two posters at AAD 2025 highlighted different challenges of managing patients with HS.
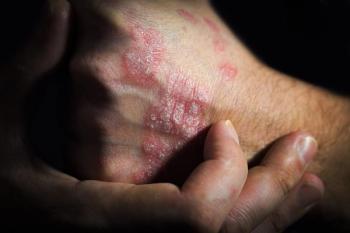
AAD 2025. New research indicates that dual inhibition of IL-17A and IL-17F with bimekizumab maintains high rates of complete skin clearance for up to 5 years.

More than half (55.4%) of roflumilast-treated patients achieved a score of 0 or 1 vs 19.8% in the vehicle group on the Worst Itch-NRS numeric rating scale.

Participants in the INTEGUMENT-1 and INTEGUMENT-2 phase 3 trials had failed treatment with topical corticosteroids and calcineurin inhibitors or crisaborole.

The findings highlight the potential of the interleukin-23 inhibitor to address a notoriously difficult-to-treat manifestation of psoriasis.

The findings from the long-term extension ADjoin study will be presented at the 2025 American Academy of Dermatology (AAD) Annual Meeting.

Preview the posters and abstracts covering atopic dermatitis advances from Arcutis, Incyte, Lilly and Sanofi/Regeneron.

There are 3 classes of nonsteroidal topical therapies for atopic dermatitis that target the specific underlying pathophysiology of the skin disease. Shahriari provides a primer as physicians prepare for AAD 2025.

The FDA set the Prescription Drug User Fee Act (PDUFA) date for December 16, 2025.
Investigators concluded that dupilumab reduced exacerbation rates, improved lung function, and decreased systemic corticosteroid use in patients with COPD driven by type 2 inflammation.

The FDA approval of neffy 1 mg follows the earlier approval of the 5 mg version in August 2024.
AAAAI 2025: Data from the ANCHOR-1 and ANCHOR-2 phase 3 trials show improvements in nasal polyp size and obstruction for depemokimab with twice-yearly dosing vs placebo.
The first low-dose rivaroxaban generic tablets to be FDA approved lead a field crowded with applications for formulations of all the higher doses.
New evidence-based guidance for bowel prep considers patient preference and history and updates timing of prep regimen, dietary restrictions, and follow-up recommendations.

Overweight individuals in the highest dose group with weight loss at day 28 continued to lose weight at the 2-week follow-up, with metabolic changes mimicking ketogenic effects.

Your daily dose of the clinical news you may have missed.

Posing an unparalleled threat of premature disease and death globally, the unchecked rise in overweight and obesity requires an urgent 5-year action plan, experts say.
The impact of food elimination diets on medication previously was unknown, according to new research presented at AAAAI 2025.

Axsome Therapeutics reported that the FDA feedback supports the company's regulatory package for a sNDA for the dextromethorphan/bupropion combination.

Your daily dose of the clinical news you may have missed.