
mRNA1345 (mResvia) is now indicated for adults aged 18 to 59 years with underlying conditions including cardiovascular, respiratory, and endocrine disorders.

mRNA1345 (mResvia) is now indicated for adults aged 18 to 59 years with underlying conditions including cardiovascular, respiratory, and endocrine disorders.

RAD 2025: Renowned AD investigator Kircik provides the history behind the classwide black box warning, highlights JAK inhibitor efficacy, and toplines exciting AD research.

Cycling and mixed cycling were associated with a reduced risk of all-cause dementia, including of young onset, as well as increased hippocampal volume.

These 14 recommendations from the American College of Lifestyle Medicine comprise the core of the first-ever guideline centered on lifestyle change as a first-line intervention.
High discontinuation rates as well as lower maintenance dosages of semaglutide and tirzepatide were among the independent predictors of lower real-world weight loss.

RAD 2025: Chovatiya toplines shifts in dosing strategies for topical, oral, and injectable AD therapy observed in both clinical trials and clinical practice.

Novel guidelines include specific strategies that support the prescription, implementation, and monitoring of lifestyle changes to reach glycemic control and, possibly, disease remission.
Both investigational vaccines induced strong immunity across all antigens and researchers reported no new safety signals, supporting phase 3 development.

Vaccination rates among US adults are roughly half that of pediatric populations, exposing older adults to risk for disease and complications, according to a new analysis.

The novel triple fixed-dose combination of telmisartan, amlodipine and indapamide, is the first of its kind approved for initial treatment of hypertension.

The preventive monoclonal antibody is designed to protect infants against RSV infection through 5 months and is administered at the same dose, regardless of weight.

Clinicians learn to recognize the hidden psychosocial burdens of atopic dermatitis, emphasizing comprehensive care beyond skin symptoms for better patient outcomes.

RAD 2025: World expert on the pathophysiology of itch Gil Yosipovitch, MD, answers questions on the disease of chronic itch and on the expanding options for targeted treatment.

RAD 2025: Mobile health data reveal a troubling decline in systemic treatment for moderate to severe atopic dermatitis, underscoring issues in access and clinical inertia.

RAD 2025: Adults and adolescents treated with nemolizumab experienced rapid onset of action and robust improvements in pruritis, sleep, and quality of life up to 2 years.

RAD 2025: Mona Shahriari, MD, underscored the cumulative life impact of atopic dermatitis, reminding clinicians that there is always far more to the condition than meets the clinical eye.

RAD 2025: In the TRuE-AD3 study, participants aged 2-6 and 7-11 years spent nearly half of the long-term treatment period free of medication.

RAD 2025: Nemolizumab as adjunctive therapy to topical steroids with/without topical calcineurin inhibitors provided rapid relief in moderate-to-severe atopic dermatitis.

RAD 2025: Safety and tolerability of roflumilast cream 0.15% was demonstrated in a pooled subgroup analysis of phase 3 study participants unresponsive to or intolerant of previous topical therapy.

RAD 2025: This unique study design simulates the real-world use of JAK inhibitors in tailored treatment regimens with flexibility to manage AEs.

RAD 2025: Arcutis Biotherapeutics will present promising long-term data on roflumilast cream, focused on effectiveness and safety for treating atopic dermatitis in young children.
A study reveals low clinician engagement in digital cognitive assessments for dementia in primary care, highlighting workflow and other barriers to early detection.
Social determinants including partner status, smoking history, and BMI were significantly associated with reduced access to effective hormone therapy for menopausal women.

Breakthrough device designation for the multicancer detection test is supported by clinical validation data demonstrating an overall specificity of 98.6% and sensitivity of 60%.

A new study of a circulating tumor DNA-based screening test revealed promising sensitivity and specificity for for CRC, but only 12.5% sensitivity for advanced neoplasia.

Nasus Pharma says promising phase 2 results for FMXIN002 show statistically significantly faster absorption than traditional autoinjectors and are published in global journal.
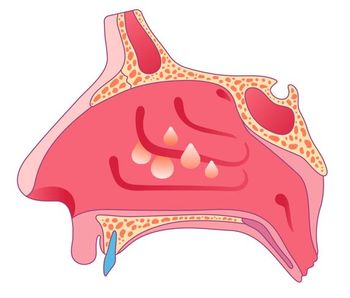
Lyra Pharmaceuticals designed the implant, which provides 6 months of continuous delivery of the anti-inflammatory mometasone furoate.

Interim results from COURAGE trial suggest combining semaglutide with muscle-preserving antibodies significantly enhances fat loss while preserving lean mass in obesity treatment.

Moderna's mRNA-1283 vaccine gains FDA approval for high-risk groups, following a significant shift in COVID-19 vaccination guidelines by federal health agencies.

Kennedy over 2 weeks has introduced changes in vaccination recommendations and research policy that undermine already tenuous public trust and understanding.