
Your daily dose of the clinical news you may have missed.

ACC.24: Icosapent ethyl was associated with reduced rate of MACE at all baseline levels of Lp(a) in participants with elevated TG and at high risk for CVD.

Your daily dose of the clinical news you may have missed.

Specifically, the topical analgesics, available over the counter, may contain higher than labeled concentrations of lidocaine, according to the agency.

The FDA approved the oral medication to treat anemia in adult patients with chronic kidney disease on dialysis for at least 3 months.

Your daily dose of the clinical news you may have missed.
Longer duration of remission was associated with a greater reduction in CKD and CVD risk, according to a post hoc analysis of the Look AHEAD study.

Your daily dose of the clinical news you may have missed.

The mRNA bivalent vaccine candidate produced a "more robust" immune response vs Spikevax and particularly in adults over the age of 65 years, said Moderna.

The once daily tablet, VK2375, is headed to a phase 2 trial later this year and is an oral version of Viking's investigational injectable GLP-1/GIP mimetic currently in phase 2.
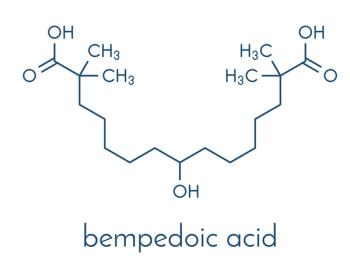
ACC.24: Data from an analysis of the CLEAR Outcomes trial showed bempedoic acid was well-tolerated and reduced CV events in Hispanic/Latinx and non-Hispanic/Latinx participants.

CLEAR Outcomes PI Steven Nissen, MD, links findings from the pivotal trial with labeling updates that make bempedoic acid the only nonstatin agent approved to reduce CV risk.

The novel SGLT-2/SGLT-1 inhibitor reduced risk of stroke, MI, and demonstrates significant antiplatelet activity in post-hoc analyses of SCORED phase 3 trial.
ACC.24: Findings showed a disproportionate distribution of CAC screening that favors White, educated, affluent, English-speaking individuals.

ACC.2024: In a cohort of people at high risk of ASCVD, three-quarters achieved target LDL-C levels at 6 months and 12 months while using a remote algorithm-directed program.

Your daily dose of the clinical news you may have missed.
Overall, a 33% increased risk of major bleeding was seen, with risk highest during the first 30 days of concomitant use and persistent, but lower, after 6 months.

For the first time, 2 drugs recommended as first line treatment for PAH are combined in a single oral once-daily therapy, which could reduced burden of care.

Bempedoic acid is now the only non-statin therapy indicated for primary CVD prevention, with potential to expand treatment access to millions, said Esperion Therapeutics.

CMS says Medicare Part D plans will cover semaglutide for individuals with overweight/obesity who have preexisting CVD, a first after 40 years of legal prohibition.