
Respiratory vaccine R & D at Moderna, Merck, and Pfizer is focused on mRNA technology, added protection for vulnerable populations, and combination shots.

Respiratory vaccine R & D at Moderna, Merck, and Pfizer is focused on mRNA technology, added protection for vulnerable populations, and combination shots.
Submission based on results from the phase 3 QUASAR induction study, which showed statistically significant and clinically meaningful improvements in symptoms.
New research highlights another benefit of COVID-19 vaccination, reported researchers.

Michael A. Weber, MD, an investigator in the PRECISION study, discusses the newly FDA approved drug aprocitentan (Tryvio), and its promise for helping patients with treatment-resistant hypertension.
Cologuard Plus bests previous iteration and demonstrates greater sensitivity and specificity for any CRC relative to fecal immunochemical tests.

Your daily dose of the clinical news you may have missed.
LEGEND phase 2 interim analysis findings include significant decreases in HbA1c in poorly controlled T2D and positive effects on several markers of liver injury.

Your daily dose of the clinical news you may have missed.
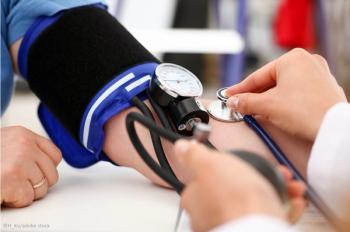
Aprocitentan is the first antihypertensive drug approved in more than 30 years that achieves its effect through a unique therapeutic pathway.
The novel dual NK-1,3 receptor antagonist demonstrated statistically significant reduction in the frequency of moderate-to-severe VMS vs placebo.
The novel 21-valent pneumococcal vaccine remains on track for the FDA PDUFA date of June 17, 2024; Merck adds this new data to the body of positive evidence.
Measles cases are edging up in the US again as MMR vaccination rates continue to decline. Have parents forgotten the Ohio outbreak of 2022? Have you? Find out, here.

Your daily dose of the clinical news you may have missed.
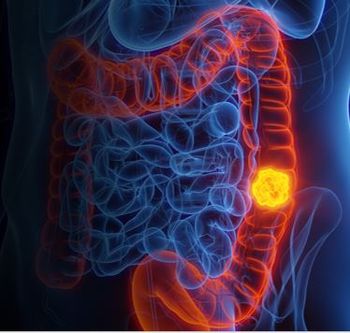
Findings from the ECLIPSE clinical trial showed the blood-based cancer screening test detects 83% of people with CRC, with a specificity of 90%.
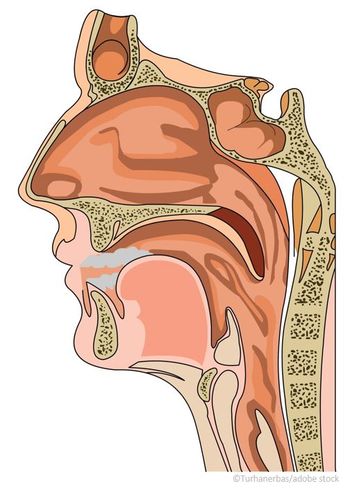
Now FDA approved, Optinose’s Xhance is the first nonsurgical option for chronic rhinosinusitis both with and without nasal polyps.

Your daily dose of the clinical news you may have missed.

Resmetirom is the first-ever drug to be approved anywhere for management of the progressive liver disease.

Your daily dose of the clinical news you may have missed.

THARROS is the first phase 3 RCT to evaluate an inhaled triple combination therapy for reduction of adverse cardiopulmonary events in people with COPD, says AstraZeneca.