At ESC 2025, the STEER study showed semaglutide reduced risk of heart attack, stroke, or death by 57% vs tirzepatide in adults with obesity and cardiovascular disease, complementing prior trial data.

At ESC 2025, the STEER study showed semaglutide reduced risk of heart attack, stroke, or death by 57% vs tirzepatide in adults with obesity and cardiovascular disease, complementing prior trial data.
.jpg?w=350&fit=crop&auto=format)
The FDA has accepted a New Drug Application for ensitrelvir, with a PDUFA action date set for June 16, 2026.

Your daily dose of the clinical news you may have missed.

The FDA has cleared Eisai and Biogen’s Leqembi Iqlik, the first at-home subcutaneous autoinjector for maintenance therapy in patients with early Alzheimer disease.

Your daily dose of the clinical news you may have missed.

Primary care clinicians see a higher share of visits for depression than mental health professionals largely because they know their patients so well, Lovins said.
Based on the FAQ section in the ACC's 2025 Concise Clinical Guidance on vaccination in adults with CVD, this short slideshow also offers recommended answers.

Teva announced approval of the generic formulation of Saxenda, which could help expand access to the extremely popular class of antiobesity medications.

Your daily dose of the clinical news you may have missed.

Teresa Lovins, MD, highlights a study that showed a 14% increase in rates of safety planning within 2 weeks of a negative screening led to a 25% decrease in attempted suicides.

This week’s podcast episode covers CRC screening, GLP-1 eligibility in youth, women’s heart health, FIT outreach, and ultraprocessed food risks.

In a trial of over 112 000 US adults, researchers found that 14% started a GLP-1 receptor agonist after bariatric surgery.

The ACC provides evidence-based guidance on improving vaccine uptake in vulnerable patients, addressing vaccine hesitancy, and improving overall vaccine access.
ESC 2025: The first global investigation of evidence for the potential positive connection is encouraging, but more research is needed to understand causality.

Your daily dose of the clinical news you may have missed.
.jpg?w=350&fit=crop&auto=format)
Infectious disease and family medicine experts share guidance on vaccine policy changes, patient communication, and boosting vaccine confidence.

Teresa Lovins, MD, sees at least 1 or 2 patients a week who have suicidal ideation. Too many of them are at risk for an attempt within a month of those visits.
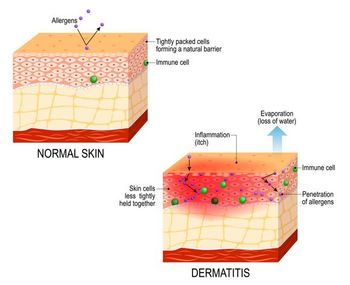
MRGPRX2 antagonism is the only known mechanism of action designed to modulate mast cells and sensory neurons, both key drivers of lesions and itch in AD.

New ACC guidance emphasizes the critical role of vaccinations in reducing infection risks and improving outcomes for adults with cardiovascular disease.
A survey by The Physicians Foundation found most US clinicians feel that faulty medical information has increased in the last 5 years and half say it has increased significantly.