
Your daily dose of the clinical news you may have missed.
.jpg?w=350&fit=crop&auto=format)
Discover the latest advancements in atopic dermatitis treatment, including new topical agents and biologics, enhancing care for patients with moderate to severe conditions.

Mental health issues affect half the population at some point during a lifetime. Teresa Lovins, MD, says FPs are often the first to learn that "something is going on."

The FDA removed the prior requirement for a person who is already diagnosed with a cardiovascular disease.
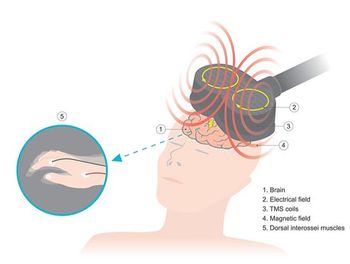
One-Day TMS condenses transcranial magnetic stimulation therapy treatment from a daily month-long process to 1 day, with rapid and durable results, NeuroStim says.

Patients taking orforglipron achieved significant weight loss, hemoglobin A1c reduction, and cardiometabolic benefits in the phase 3 ATTAIN-2 study, supporting upcoming global regulatory submissions.

People with obesity may shun basic primary care for fear of shaming, lectures, inhospitable equipment, and simple lack of respect from clinicians, authors found.

Your daily dose of the clinical news you may have missed.
.jpg?w=350&fit=crop&auto=format)
Linda Stein Gold, MD, reviews AAD’s 2025 updates on new topical and biologic options for adult atopic dermatitis.
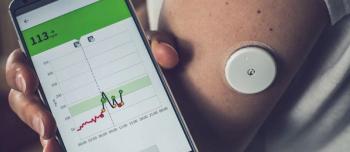
The combination of the Dexcom Stelo glucose biosensor with an AI platform provides real-time data on how food, exercise, stress, and sleep affect the body.

ACOG CEO: “In the face of misinformation and vaccine hesitancy, a strong, evidence-based recommendation...from a trusted clinician can go a long way."

The protective effect of the diet was strongest in the highest-risk group, those homozygous for the APOE4 gene variant, suggesting that diet may help offset genetic risk.

Your daily dose of the clinical news you may have missed.

More than 90% of adults making nonurgent ED visits had missed at least 1 CDC-recommended vaccine but many were willing to catch up on all of them while there.

Infectious disease expert shares strategies for PCPs to counter vaccine myths, navigate policy changes, and build patient trust.

GLP-1 use was associated with lower overall cancer risk, with the strongest associations observed for endometrial and ovarian cancers and meningioma.
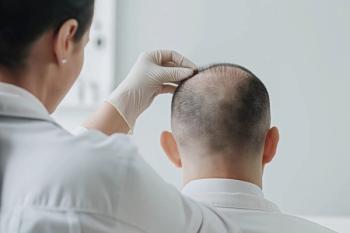
AbbVie reports that upadacitinib met primary and secondary endpoints, with up to 55% of patients achieving at least 80% scalp hair coverage at 24 weeks.
Z-1018, which combines a glycoprotein E (gE) antigen and proprietary adjuvant, was evaluated in adults aged 50 to 69 and the 100 µg dose will advance to part 2 of the trial.

Your daily dose of the clinical news you may have missed.
.jpg?w=350&fit=crop&auto=format)
Shagun Bindlish, MD, board member of the American Diabetes Association, discusses challenges to diagnosing and treating venous disease in patients with obesity.