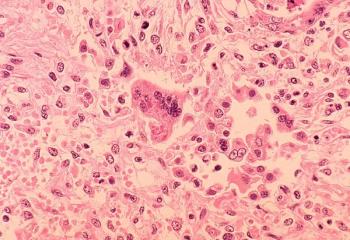
The 2025 measles outbreak in Texas has reignited discussion surrounding vaccine-preventable diseases and the importance of maintaining high immunization rates.

The 2025 measles outbreak in Texas has reignited discussion surrounding vaccine-preventable diseases and the importance of maintaining high immunization rates.
A rural west Texas "undervaccinated" Mennonite community is the epicenter of the worst measles outbreak in the state in 30 years, the state health department reported.
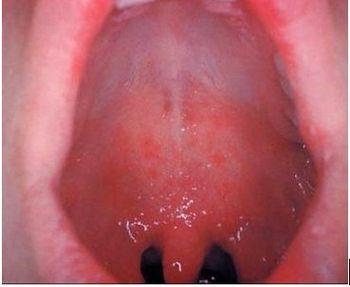
A case of measles is a very rare diagnosis in the 21st century, but as MMR vaccination rates fall, the cases climb. Would you recognize the signs & symptoms?

Here, the 5 key signs and symptoms that characterize measles in the order of appearance.

Your daily dose of the clinical news you may have missed.

Researchers found that COVID-19 had a higher long-term mortality than influenza and RSV in veterans—underscoring vaccination’s vital role.

The study of S-337395 showed an 88.9% reduction in RSV viral load and symptom improvement in the highest dose group, with no severe adverse events reported.

These test panels allow clinicians to diagnose multiple STIs from a single specimen, with results available in about 20 minutes.

Encouraging patients to reach out with questions may lead more to roll up their sleeves this season, Tochi Iroku-Malize, MD, told Patient Care.
The novel therapeutic demonstrated a strong safety profile and showed efficacy in reducing symptoms during the early stages of treatment.
The FDA approved the addition of Guillain-Barré syndrome warnings for Abrysvo and Arexvy, citing postmarketing data suggesting an increased risk.

The bird flu threat remains low, according to public health authorities, but awareness and vigilance will help keep it that way. Here are 9 things you need to know.

The first death of an American from the H5N1 virus was reported in Louisiana on Monday, though authorities maintain the risk of widespread infection remains low.
Sanofi and SK bioscience expand collaboration on pneumococcal conjugate vaccines, including a 21-valent candidate for pediatric populations.

Your daily dose of the clinical news you may have missed.

Your daily dose of the clinical news you may have missed.

Your daily dose of the clinical news you may have missed.

Your daily dose of the clinical news you may have missed.

Your daily dose of the clinical news you may have missed.
Comments on the draft recommendation can be submitted through December 23, 2024.

At least 1 accurate UTI symptom was found on most of the 331 websites reviewed, but nearly all (80%) included at least 1 inaccurate or misleading one.

The recommendations from WikiGuidelines are the first for UTI prevention, diagnosis, and management in over a decade.
Analysis of surveillance data revealed a decline in use of antiviral medications for children hospitalized with influenza and prevalent underprescribing in outpatient settings.
More than 2.4 million STIs were reported in the US in 2023 — a 1.8% decrease from 2022, according to the CDC's annual STI report.
The FDA cleared Novavax to start enrolling the planned phase 3 trial after a safety concern was found to be unrelated to the combination vaccine.