COVID-19, influenza, and RSV will be the topics of a different types of sessions examining the history, new diagnostics, prevention, and future treatments during IDWeek 2023.
Coadministration of RSV, Influenza Vaccines is Safe and Effective in Older Adults, Study Finds

COVID-19, influenza, and RSV will be the topics of a different types of sessions examining the history, new diagnostics, prevention, and future treatments during IDWeek 2023.
Many patients with moderate-to-severe asthma may not be using inhaled corticosteroids along with rescue medications to avoid serious disease exacerbations.
Influenza B is the diagnosis and the patient wants to start antiviral therapy. Factoring in CDC guidance, patient preference, and price, which of the 4 options makes most sense?

Your daily dose of the clinical news you may have missed.

Your daily dose of the clinical news you may have missed.

Your daily dose of the clinical news you may have missed.

Waist-to-hip ratio was more powerfully and consistently associated with risk of all-cause mortality than BMI or FMI, but can it replace BMI as a clinical indicator?

Meta-analysis: Among persons with symptoms of long COVID, respiratory and exercise training were effective but pooled information on safety was "uncertain, imprecise."

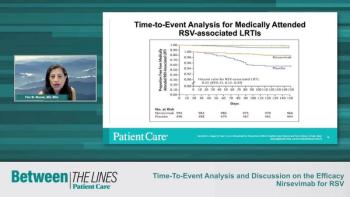
Experts conclude their discussion on the efficacy and safety study of nirsevimab for Respiratory Syncytial Virus (RSV) commenting on possible dosing strategies and key takeaways from this study.

Experts discuss the results of the safety and efficacy study of nirsevimab for Respiratory Syncytial Virus (RSV) commenting on the adverse events observed in the test and placebo groups.

Experts discuss how infant weight related to efficacy outcomes of nirsevimab for Respiratory Syncytial Virus (RSV), providing their clinical expertise on why this may have occurred and what improvements can be made to mitigate these differences.

Experts discuss data relating to the strains of Respiratory Syncytial Virus (RSV) and how clinicians should consider them in relation to the efficacy of nirsevimab.
Flor M. Munoz, MD, MSc, and Tina Tan, MD, FAAP, FIDSA, FPIDS, continue their discussion on the results of nirsevimab for Respiratory Syncytial Virus (RSV) and offer insights on how this may change therapeutic approaches in the future.

Tina Tan, MD, FAAP, FIDSA, FPIDS, and Flor M. Munoz, MD, MSc, discuss the initial efficacy data of the study on nirsevimab for Respiratory Syncytial Virus (RSV) and how it compared to placebo.
Flor M. Munoz, MD, MSc, and Tina Tan, MD, FAAP, FIDSA, FPIDS, discuss the patient population of the safety and efficacy study on nirsevimab for Respiratory Syncytial Virus (RSV).

Tina Tan, MD, FAAP, FIDSA, FPIDS, presents the background, objectives, and methods of a recent study on the efficacy and safety of nirsevimab for Respiratory Syncytial Virus (RSV) in late-preterm and term infants.
Flor M. Munoz, MD, MSc, and Tina Tan, MD, FAAP, FIDSA, FPIDS, discuss the prevalence of Respiratory Syncytial Virus (RSV) and prior preventative gaps. They also discuss the recent FDA-approval of nirsevimab for late-preterm and term infants.
Tina Tan, MD, FAAP, FIDSA, FPIDS, and Flor M. Munoz, MD, MSc, introduce the objectives of their discussion and background information on Respiratory Syncytial Virus (RSV) and nirsevimab.

Reduced exacerbations, improved lung function, increased QoL, and less use of systemic corticosteroids underscore dupilumab's effects against type 2 inflammation.

As COVID-19 hospitalizations increase at the opening of the respiratory virus season, the first "seasonal" vaccine against the virus is ready for roll-out, says the FDA.

"Keep the vaccination conversation going this year, even though your patients, and probably you, too, have grown tired of the issues and your team is worn out."

A daily dose of clinical news on Patient Care you may have missed.

The FDA approved Abrysvo for use in pregnant individuals to prevent LRTD and severe LRTD caused by RSV in infants from birth through 6 months of age.

Approximately 80% of infants hospitalized with an RSV-related illness during the 2022 seasonal peak did not have underlying medical conditions, according to new study.
Despite a 20-pack year smoking history and frequent respiratory exacerbations, spirometry is preserved in this group of heavy smokers.