
Dagogo-Jack reviewed alarming data on the damage prediabetes can inflict even decades before a diabetes diagnosis and offered guidance to the internal medicine audience.

Dagogo-Jack reviewed alarming data on the damage prediabetes can inflict even decades before a diabetes diagnosis and offered guidance to the internal medicine audience.

ACP 2025. A quick look at 2 topics in dermatology rounded out a Multiple Small Feedings of the Mind session at the 2025 American College of Physicians annual meeting.

ACP 2025. Ruth Etzioni, PhD, discussed evidence-based approaches to screening for patients at average risk, start and stop criteria, MRI for follow-up screening, and other topics.

ACP 2025: Irl B Hirsch, MD, reviewed findings from 8 papers published in 2024 that he believes have made or will make a difference in the practice or understanding of the clinical area.

ACP 2025. Thomas Martens, MD, Gregg Simonson, PhD, and Libby Johnson, RDN, LD, CDCES explained the benefits of CGM, how to interpret data, and then adjust treatment accordingly.
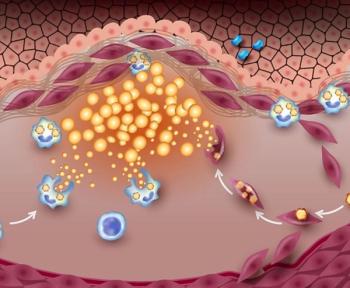
Findings of the EXSTROM trial may help elucidate the mechanism by which low-dose colchicine prevents MI and stroke in adults with established CAD, experts suggest.

In treatment-resistant PTSD, TSND-201 was associated with statistically significant symptom improvement as early as day 10 in the phase 2 IMPACT-1 trial.

ACC.25. The results seen with the only oral GLP-1 mimetic reflect "a profound clinical impact" for adults at high risk for MACE who are averse to injectable therapy.

Lepodisiran at 400 mg maintained reductions of Lp(a), an inherited risk factor for cardiovascular disease, at ~90% at 1 year and ~75% at 1.5 years, Eli Lilly reported.
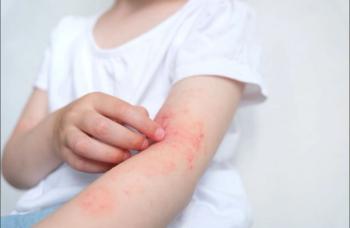
Dupilumab significantly improved measures of severe AD, including EASI and IGA, as well as pruritis in pediatric populations aged 6 months to 18 years vs placebo.

In the pediatric population aged 0 to 19 years, exposure to fentanyl was through intentional use by two-thirds, investigators found.
The 2025 measles outbreak is predominantly affecting children, but measles is highly contagious and adults remain at risk, especially if they have not received the MMR vaccination.

An estimated 3000 Americans progress daily from mild to moderate or severe stages of Alzheimer's disease, making timely diagnosis imperative, Lilly said.

Mean reduction in HbA1c was nearly 1% after transition from MDI plus CGM to Omnipod 5 in the first randomized trial to evaluate the shift in mode of insulin delivery.


A new analysis reveals significant gaps in diagnosis of and treatment for psychiatric disorders in adults with COPD and calls for larger, longitudinal studies.

GSK is partnering with the UK Dementia Research Institute and Health Data Research UK to further investigate the potential for the RZV vaccine to mitigate cognitive decline.

Data from the phase 2 VENTURE-Oral Dosing Trial of VK2735 is expected in the second half of the year, the company said.

Data on use of lecanemab in real world clinical settings will come from a neurologic case series review and a retrospective chart review of long-term use, both in early AD.

More than one-third of study participants with TRD had failed to get relief from 4 or more antidepressants, calling treatment experiences a "trial and error" process.

Raj Chovatiya, MD, PhD, MSCI, actually said symptoms "never" lie so, particularly in patients with skin of color, if skin signs aren't clear, ask about the patient's experience.

For the same level of baseline health, the increase in risk related to each of 8 key CV risk factors appears significantly higher in women than in men.

The reductions observed in all-cause mortality (49%) and MACE (39%) in high-risk patients compared with statin monotherapy could be practice changing, researchers said.

AAD 2025: Raj Chovatiya, MD, PhD, MSCI, well-known physician scientist, says underrepresentation in clinical trials of those with skin of color precludes an evidence-based answer.

In a small study of adults genetically predisposed to early-onset dementia, anti-amyloid treatment to remove plaque reduced risk of developing symptoms by 50%.

The greatest risk for developing cancer faces residents of South Dakota, where radon levels are the nation's highest, and ranks for alcohol consumption and obesity are poor.

The IL-23 inhibitor is the only drug in the class available with both subcutaneous and intravenous administration options for treatment induction, according to J&J.

Raj Chovatiya, MD, PhD, MSCI, discusses the complex and widely variable influences that affect the manifestations and burden of atopic dermatitis across populations.

AAD 2025: Chovatiya discusses whether melanin concentration plays a role in skin physiology and cautioned against typing with factors that are not true biologic constructs.

In individuals with skin of color with AD, lebrikizumab was associated with improved QoL and importantly with high rates of patient-reported treatment satisfaction.