
The lead investigator of the largest clinical trial program in people with CAH discusses the condition, its burden, and the novel drug just approved that will change lives.

The lead investigator of the largest clinical trial program in people with CAH discusses the condition, its burden, and the novel drug just approved that will change lives.

Clesrovimab, if approved, would be the only immunization for both healthy and at risk infants using the same dose. FDA set A PDUFA date of June 2025.

Arcutis Biotherapeutics announced submission of the sNDA today, citing the the supporting positive data from the phase 3 INTEGUMENT-PED and INTEGUMENT-OLE trials.

Approval for the topical nonsteroidal therapy, based on data from the phase 3 ADORING clinical trial program, helps fill a gap in current offerings for atopic dermatitis.

Auchus details findings from the unique study of the CRF-1 antagonist crinecerfont, the first new therapy for CAH approved in 70 years.

Crinecerfont approval makes available the first new treatment for the life-altering endocrine disorder in more than 70 years, according to Neurocrine Biosciences.

The new nonsteroidal topical treatments for atopic dermatitis are targeted and modify the disease process, explains thought leader Shahriari in this year's video series.
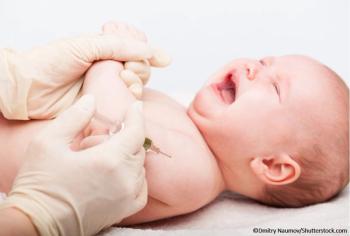
Moderna paused clinical research on the pediatric shot indefinitely in September after reports in July of severe adverse reactions in infants aged 5 to 8 months.

An analysis of patient reported outcome measures from 3 pivotal phase 3 RCTs of atogepant 60 mg demonstrates efficacy across functional domains as well as for migraine prevention.

An MDF stethoscope with leopard print tubing? A digital highlighter/scanner that translates and talks back? Check out these and 8 more uncommon gifts for health care professionals.

Americans' rating of the quality of health care available in the US has hit its lowest in 24 years, according to a new Gallup survey; here's what they think.

How does alcohol figure in to American holiday celebrations? We checked with Alcohol.org for this quiz. Are answers what you expect?

Dupilumab significantly reduced signs and symptoms of AD in children aged 6 months to 5 years with concomitant asthma, allergic rhinitis, and food allergy.

The CGRP inhibitor fremanezumab reduced both monthly migraine and monthly headache days among children aged 6 to 17 years with a favorable safety profile.

"Twincretin" tirzepatide-treated participants in SURMOUNT-5 lost a mean 50 lbs vs an average loss of 33 lbs for those treated with semaglutide.

Aquestive Therapeutics announced positive feedback from the FDA this week that keeps NDA planning for the novel epinephrine product on solid ground.

Holiday health care gift-giving season is here and we've done some window shopping for our primary care readers. Take a breath and scroll through our top 10 suggestions for 2024.

At least 1 accurate UTI symptom was found on most of the 331 websites reviewed, but nearly all (80%) included at least 1 inaccurate or misleading one.

Millions of US adults could benefit from semaglutide treatment for diabetes, obesity, and/or CV disease but insurance isn't guaranteed and the drug is costly.
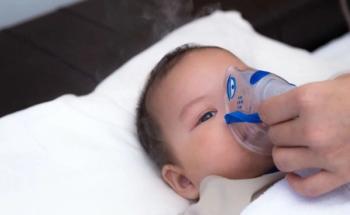
Analysis of surveillance data revealed a decline in use of antiviral medications for children hospitalized with influenza and prevalent underprescribing in outpatient settings.

Geriatric psychiatrist and neurocognitive researcher George Grossberg, MD, highlights the mechanism of action for the new dextromethorphan/bupropion combination.

Physical activity of any level before and after a diagnosis of dementia was associated with at least a 20% lower risk for all-cause mortality across dementia subtypes.

Lp(a) is a genetically-driven and independent risk factor for CVD with no approved treatments. Lilly is advancing muvalaplin and has some good competition.

Among current smokers, 71% had never spoken a clinician about screening and among those who had quit, 75% had never had a conversation.

AHA 2024. Findings from the BPROAD trial help fill a gap left by similar studies on the ideal target SBP for adults with type 2 diabetes, said study authors.

The EMA recommendation excludes from treatment adults who are homozygous for the ApoE ε4 gene, a decision that the US Alzheimer's Association does not support, said the president/CEO.

About 40% of people who have T1D are not aware of it until they experience an extreme health event; screening in primary care can help reduce the risk, support families.

A quick review of the scope of diabetes in the US and of the gaps in equitable access to care supports the annual call to action from the International Diabetes Federation.

Michigan Medicine researchers found that at all age levels, women with known/suspected OSA were more likely than men to be diagnosed with dementia.

The 11 words and phrases, among others, are often automatic responses from clinicians in highly emotional settings of severe illness. Find 11 alternatives, here.