Data from a study performed during the first season the shot was available expand on clinical trial evidence and support the safety of RSVpreF, authors wrote.

Data from a study performed during the first season the shot was available expand on clinical trial evidence and support the safety of RSVpreF, authors wrote.

Tirzepatide-treated participants were 3 times more likely than those treated with semaglutide to reach weight loss of 15% or greater, the head-to-head study found.
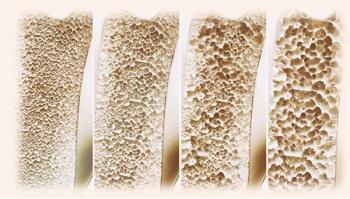
The preserved bone mass observed in participants taking liraglutide and exercising was seen despite weight reduction levels similar to outcomes with semaglutide and tirzepatide.

Donanemab, now 1 of 2 antiamyloid medications available, is unique based on evidence that it can be used in limited duration treatment, based on removal of plaque.

The international statement targets primary care clinicians and endocrinologists with detailed guidance on monitoring disease progression and providing education.
The novel vaccination is recommended as an option for adults aged 65 years and older who have not received a pneumococcal conjugate vaccine.

Neurocrine Biosciences made the announcement and said, if approved in December, crinecerfont will be the first new treatment and treatment approach to CAH in 70 years.
The standard of care for congenital adrenal hyperplasia has not changed in more than 60 years nor have the challenges it presents been overcome.

Investigational treatments for congenital adrenal hyperplasia are focused on new ways to manage symptoms without the need for supraphysiologic doses of glucocorticoids.
Current therapies fall short of quelling the long-term exposure to and adverse effects of excessive androgen levels experienced by adults with CAH.

The committee endorsed a universal recommendation for a single lifetime dose of RSV vaccine for adults aged ≥75 years and a risk-based recommendation for those aged 60 to 74 years.
Nearly one-third of US adults aged 60 years and older continue to take daily aspirin despite published evidence recommending against use in the absence of CVD.

ADA 2024. The risk of progression to stage 3 type 1 diabetes may be better predicted using a combination of patient and CGM data, a new study suggests.

ADA 2024. MOMENTUM trial findings presented at the ADA yesterday showed 78.1% of weight loss attributable to loss of fat vs lean mass in adults with overweight or obesity.
Fiver years after ACS, a new study shows women are less likely to be on lipid lowering therapy and to meet LCL-C target levels than men.

The grade B recommendation (moderate certainty) targets youth aged 6 years and older with a BMI at or above the 95th percentile for age and sex.

Described as a giant and a pioneer in the hypertension and metabolic fields, Bakris is also remembered so fondly by colleagues as a very special man.

The doubling of the diagnosis of chronic hypertension in pregnancy was accompanied by no increase in treatment rates, according to a new study.

Among youth aged 10 to 17 years with uncontrolled hyperglycemia dapagliflozin led to a statistically significant reduction in A1c vs placebo in the T2NOW phase 3 clinical trial.

Capvaxive is the only pneumococcal vaccine formulated specifically to protect adults 50 years and older from IPD and elicited robust immune responses in both vaccine-naïve and vaccine-experienced adults.

In part the National Academies describe long COVID as "a chronic, systemic disease state with profound consequences." The report provides a working definition.
The high potency IL-5 inhibitor can be administered every 6 months, offering a convenient alternative to other biologic agents for appropriate patients with severe asthma.

The FDA has assigned a PDUFA action date of January 25, 2025 for the monthly IV maintenance dose.
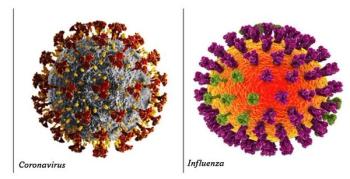
The investigational combination vaccine elicited higher immune responses vs licensed comparator vaccines in 2 independent age groups, the company said.

GSK announced today approval by the US FDA of a label expansion for the adjuvanted RSV vaccine that adds adults aged 50 to 59 years who are at elevated risk for RSV disease to the vaccine’s current indication.
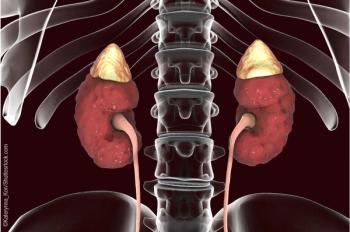
The registrational clinical trial met all endpoints related to androgen reduction and glucocorticoid dose reduction while maintaining androgen control.

Despite its limitations for use in other populations, a high BMI proved a very good predictor of dangerous fat mass index in youth aged 8 to 19 years in this study.

The over-the-counter antigen test, for use at home or at point of care, discriminates between SARS-CoV-2 and influenza A and B in 15 minutes.

mRESVIA is Moderna's second mRNA-based vaccine and will be shipped in prefilled syringes, a desirable feature that could save clinical time, prevent errors.

The dual interleukin-4, 13 inhibitor reduced COPD exacerbation rates by as much as 34% for individuals with COPD with type 2 inflammation, Bhatt explains.