COVID-19 as a cause of death fell from the #4 spot in 2022 to #10 in 2023, which may account for some of the overall decline in the US mortality rate, suggest CDC experts.

COVID-19 as a cause of death fell from the #4 spot in 2022 to #10 in 2023, which may account for some of the overall decline in the US mortality rate, suggest CDC experts.

Finerenone treatment achieved a statistically significant reduction in a composite endpoint of CV death and HF events in a population not limited to CKD in T2D, Bayer announced.

New study findings suggest that in older adults stable HbA1c within individualized target ranges over time may help reduce risk of Alzheimer disease and other dementias.

Clinical guideline recommendations for optimal timing to gauge glucocorticoid treatment effects in congenital adrenal hyperplasia require individualization. Here's why.

In young adults with congenital adrenal hyperplasia of age to transition to adult care, 25% to 50% are not successful. Here are thoughts on problems and solutions.
The lecanemab Clarity AD open label extension study returned no new safety signals and data reflect the therapy's disease modifying effects on key biomarkers.

HPV series initiation and completion was lower among adolescents seen at public vs private care settings and so was clinician guidance on vaccination in a new study.

Tirzepatide reduced the risk of severe heart failure outcomes by 38% and drove weight loss of 15.7% in adults with HFpEF and obesity, with and without T2D..

Maternal mortality nearly doubled between 2018 and 2022 in the US. The Commonwealth Fund ranks states on how well they care for women.

PK data from a self-administration study are comparable to those seen following administration by a clinician and Tmax equal at 15 minutes.
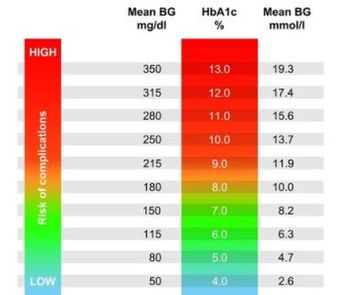
Hypoglycemia unawareness increased over time with use of sulfonylureas but decreased with use of insulin over the same period, a new study found.

The rate of ME/CFS was similar (3% to 4%) at 3 through 12 months for study participants who tested positive and negative for COVID-19.
Providing postpartum scheduling support increased the odds of a primary care visit within 4 months of childbirth among women with chronic disease and improved monitoring.
The significant rise in new GLP-1 RA prescriptions between 2011 and 2023 saw a doubling in the proportion written for obesity and a decline in those for T2D.

Limiting screen media use to 30 minutes per day or less for all family members improved mental health measures among youth aged 4 to 17 years in this randomized trial.

Among GPs, IMs, FPs, the primary reason for reluctance to treat addiction is lack of support in the "institutional environment," followed by concerns over personal skills.

Hypertension interventions led by pharmacists and community health workers were more successful in lowering BP than those led by nurses and physicians.
Ziyad Al-Aly, MD, and colleagues link 70% of the drop in cases to vaccine availability but caution the risk for long COVID remains significant.

The antigen-specific immunotherapy preserves endogenous insulin production via intralymphatic injection of recombinant GAD65 protein, according to Diamyd Medical.
Adiposity before age 60 to 64 years, beginning as early as age 20 years, was associated with adverse cardiac structure and function not explained by current BMI.

The oral GLP-1 RA was associated with weight loss of 7.3% in just 4 weeks and is designed with unique properties that will enhance multiple outcomes, Roche said.

The most concerning escalation in type 1, type 2, and gestational diabetes has been among the youngest women and girls, aged 15 to 19 years, report study authors.

An analysis of 28 000 adults with vascular disease and high-risk diabetes found daily sodium intake of ≥8 g significantly increased risk for AF by 32%.
The investigational live intranasal vaccine was safe and well-tolerated when given concomitantly with Fluzone HD and the combination outperformed the latter given by itself.
The PATHFINDER 2 and HMS-Galleri trials are proceeding as planned with more than 175 000 participants from diverse backgrounds enrolled across both.
In the mouse model of type 1 diabetes, a combined harmine/exendin-4 therapy resulted in an up to 7-fold increase in β cell numbers over 3 months, reported investigators.
Findings from the large meta-analysis provide strong support for use of both classes to reduce CV and renal disease in adults with type 2 diabetes.

The steroid-free cream was developed for long-term use and has been proven safe in an open-label extension study following successful phase 3 trials.

The agency is concerned about manufacturing processes involved in production and about the proposed indication for adults with type 1 diabetes.
Continuous statin use among adults aged 75 years and older resulted in a mean relative risk reduction in CVD of 28% without heightened safety concerns, a new study found.