
Your daily dose of the clinical news you may have missed.

Editor of Patient Care Online.

Your daily dose of the clinical news you may have missed.

At RAD 2025, pediatric dermatologist Elizabeth Swanson, MD, discussed the value of focused AD education and anticipated label expansions for several treatments.
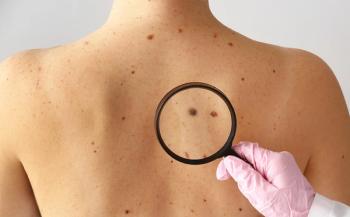
FDA-cleared DermaSensor device shows 96% sensitivity and reduces missed skin cancer diagnoses by 50% in primary care, according to new data.

At RAD 2025, Johann Gudjonsson, MD, PhD, discussed the OX-40 pathway’s role in AD, genetic links to disease risk, and promising combination therapies in development.

Valid and accessible measures are needed to monitor low-value care practices and set benchmarks for de-implementation efforts, researchers reported.

Clinical insights on cannabis and dementia, hormonal contraceptives and postpartum depression, the FDA-approved blood test for Alzheimer disease, more.

RAD 2025. Peter Lio, MD, shares details from his presentation on shared decision making when treating children with atopic dermatitis.

RAD 2025. Lebrikizumab improved skin clearance, itch, and pigmentation in patients with skin of color and atopic dermatitis, with strong safety data through 24 weeks, according to late-breaking data.

RAD 2025: Jiade Yu, MD, discussed chronic hand eczema as a distinct condition from AD, highlighting its clinical burden and emerging treatments, such as delgocitinib.
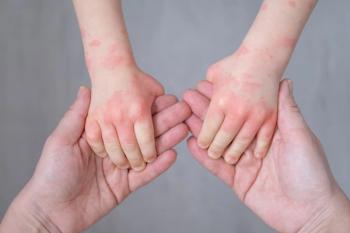
Arcutis Biotherapeutics announced today the enrollment of the first patient in the phase 2 INTEGUMENT-INFANT trial.

From primary care management of heavy menstrual bleeding to postpartum depression screening, find out what you may have missed at ACOG 2025.

RAD 2025: Explore expert insights on chronic hand eczema, its differences from atopic dermatitis, and emerging treatments in this informative video series.

RAD 2025: Many adults achieved optimal treatment targets with upadacitinib after 6 months, regardless of prior biologic therapy, according to new late-breaking data.

RAD 2025: Lisa Swanson, MD, shares insights on pediatric atopic dermatitis, addressing questions from both a dermatologist's and an allergist's perspective.

Dupilumab monotherapy showed significant improvements in atopic dermatitis symptoms in patients with skin of color, according to late-breaking data presented at RAD 2025.

RAD 2025: Researchers observed a marked reduction and low burden of viable S. aureus on lesional and non-lesional skin after 1 year of dupilumab treatment.

Donna Ryan, MD, highlights how the media has helped to reframe obesity as a chronic disease and how antiobesity drugs are transforming metabolic disease treatment.

RAD 2025: Roflumilast cream significantly improved atopic dermatitis in children and adolescents through 52 weeks, offering a safe, effective treatment option.

RAD 2025: Under lebrikizumab maintenance treatment in week 16 responders, approximately 8 out of 10 achieved almost clear skin up to 3 years.
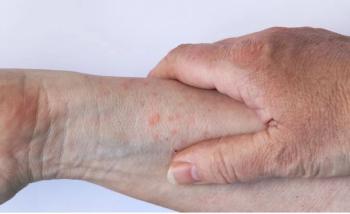
RAD 2025: More than 80% of people would want, or would consider, a test that would direct them towards a more effective therapy for their AD, according to new data.

Hear experts' takeaways from ACOG 2025 on menopause care, postpartum depression screening, and obesity management.

From HPV vaccination to PCOS management, this quiz is designed for primary care providers who want to stay ahead in women's health.

Discover the latest breakthroughs in clinical research, from at-home cancer screening to AI advancements in mammography, shaping future healthcare practices.

When asked about her 3 wishes for the future of obesity care, Donna Ryan, MD, told Patient Care that she had just one: access.

Your daily dose of the clinical news you may have missed.

ACOG 2025: Joy Baker, MD, discusses the urgent need to prioritize postpartum depression screening, normalize mental health in prenatal care, and strengthen continuity between OB-GYN and primary care.

ASCO 2025. Elinzanetant significantly reduced the frequency of VMS vs placebo in women on endocrine therapy for treatment or prevention of HR+ breast cancer.

Your daily dose of the clinical news you may have missed.

Preview of 10 new studies on managing atopic dermatitis, including biologics, treatment barriers, and innovative care strategies for clinicians.

Research reveals that the quality of foods in low-carb and low-fat diets significantly impacts heart health, emphasizing whole, plant-based options.