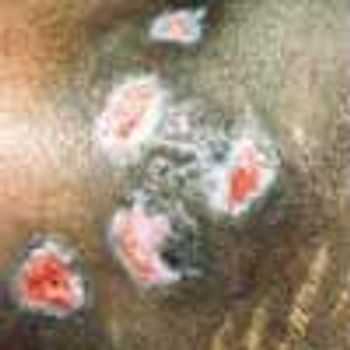
Pyoderma gangrenosum (PG) is a chronic, recurrent condition characterized by cutaneous ulceration. In half of patients, PG is associated with an underlying illness, such as inflammatory bowel disease, RA, SLE, or a lymphoproliferative disorder.

Pyoderma gangrenosum (PG) is a chronic, recurrent condition characterized by cutaneous ulceration. In half of patients, PG is associated with an underlying illness, such as inflammatory bowel disease, RA, SLE, or a lymphoproliferative disorder.

ABSTRACT: The cardinal feature of irritable bowel syndrome (IBS) is abdominal pain or discomfort associated with altered bowel habits. Because no serologic marker or structural abnormality exists, the diagnosis is based on clinical findings. A systematic symptom-based approach, including the Rome II criteria, ensures diagnostic accuracy. Determine whether a specific event-such as gastroenteritis, antibiotic use, or a food-borne illness-precipitated the IBS symptoms. Be alert for warning signs of cancer, infection, or inflammatory bowel disease, such as fever or unexplained weight loss. Only minimal laboratory testing is required; however, further evaluation may be warranted if a patient does not respond to treatment or loses weight, if the dominant symptom changes, or if other "red flags" are identified.

Adhesions (A) can form within theperitoneal cavity after abdominalsurgery, especially if there is an underlyinginflammatory condition suchas appendicitis (B) or inflammatorybowel disease. The incidence of adhesiveintestinal obstruction following alaparotomy is approximately 2%. Mostadhesive obstructions occur within 3months of the laparotomy, and 80%occur within 2 years. Adhesive obstructionstend to be more commonin children than in adults.