
Get a quick topline look at specific revisions and additions included in the ACIP's recommendations for routine immunization of those aged ≤18 years in the US.

Nirsevimab Gets FDA Advisory Committee Recommendation to Prevent RSV in Infants

FDA Advisory Group Votes in Favor of Pfizer Investigational Maternal RSV Vaccine Candidate

Get a quick topline look at specific revisions and additions included in the ACIP's recommendations for routine immunization of those aged ≤18 years in the US.
Lotteries with cash incentives and other giveaways are just one example of ways the COVID-19 pandemic changed the way US health care is handled.
Vaccination was associated with lower risks of infection, COVID-19-related illnesses, and hospitalizations due to COVID-19-related illnesses in children.

These tips from a family physician on managing misinformation in the exam room can help reduce the spread of false and misleading narratives.

The COVID-19 pandemic derailed routine immunizations and triggered a flood of vaccine misinformation beyond the COVID shot. Here, 3 ways to move forward.

AAFP president Tochi Iroku-Malize, MD, MPH, MBA, urges gentle but persistent efforts among all clinicians to get school-aged children back on vaccination track.
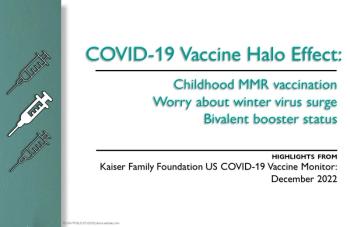
The Kaiser Family Foundation survey found a 10% drop in the proportion of adults who say MMR vaccination should be required for school attendance, and other "halo" effects.

Tochi Iroku-Malize, MD, MPH, MBA, converts the 93% immunization rate for US kindergarteners into raw numbers of children now vulnerable to vaccine-preventable disease.
If approved, nirsevimab would be the first single-dose RSV preventative option for the broad newborn and infant population in the US.

A new study points to the Pfizer shot's initially limited protection against the COVID-19 Omicron variant in those aged 5-11 years and also the the value of vaccine boosters.

The FDA amended Pfizer and Moderna EUAs for updated COVID-19 vaccines to include children as young as age 6 months as holidays and winter weather approach.

Pfizer and BioNTech submitted an application to the FDA for emergency use authorization of their omicron BA.4/BA.5-adapted bivalent COVID-19 vaccines in children aged 6 months to 4 years.

Your daily dose of clinical news you may have missed.

One in 7 parents have not discussed vaccines with their child's primary clinician during the 2-year pandemic period, according to a CS Mott Children's Hospital poll.
Overall flu activity is at a 10-year high, according to the CDC's most recent influenza surveillance report, while US hospitals are already overwhelmed.

The overall US COVID-19 vaccination picture has both bright spots and dark areas. Get a quick look at details from the most recent KFF Vaccine Monitor survey.

The FDA amended EUAs for omicron-adapted vaccines from both Pfizer and Moderna, authorizing the shots for children and adolescents.

Refractory adult misbeliefs about vaccine safety have increased reluctance to vaccinate US children aged 5-11 years, according to an Annenberg Public Policy center survey.

Pediatric COVID-19 vaccination can be tricky as vaccine antigen levels vary by age groups. These 3 patient scenarios may help clarify.